Publications
*Click on the image to take you to the online article
Manas Ranjan Panda, Sally El Meragawi, Meysam Sharifzadeh Mirshekarloo, Wanqing Chen, Mahdokht Shaibani, Mainak Majumder. “Acidity-Aided Surface Modification Strategy to Enhance In Situ MnO2 Deposition for High Performance Zn-MnO2 Battery Prototypes” Small (2024) 2311933.
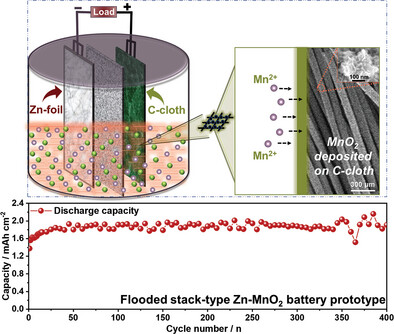
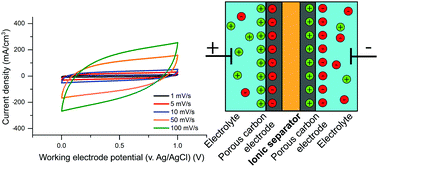
Zn–MnO2 batteries offer cost-effective, eco-friendly, and efficient solutions for large-scale energy storage applications. However, challenges, like irreversible cathode reactions, prolonged cyclability, and electrolyte stability during high-voltage operations limit their broader application. This study provides insight into the charge–discharge process through in situ deposition of active β-MnO2 nanoflakes on a carbon-based current collector. The study elucidates the effect of pH and electrolyte concentration on chemical conversion reactions with Zn, in particular focus on their impact on the two-electron MnO2/Mn2+ reaction crucial for high voltage operation. The electrolyte, characterized by being relatively lean in Mn2+ and with a targeted low pH, enables extended cycling. This research achieves greater cycling durability by integrating a carbon-based cathode current collector with high density of structural defects in combination with cell architectures suitable for large-scale energy storage. A flooded stack-type Zn–MnO2 battery prototype employing the optimized electrolyte demonstrates a high discharge voltage (≈2 V) at a substantial discharge current rate of 10 mA cm−2. The battery exhibits an impressive areal capacity of ≈2 mAh cm−2, maintaining ≈100% capacity retention over 400 cycles. This research establishes a promising practical, and cost-effective cathode-free design for Zn–MnO2 batteries, that minimizes additional processing and assembly costs.
Declan McNamara, Mahdokht Shaibani, Mainak Majumder, Matthew R. Hill “A Nanoporous Permselective Polymer Coating for Practical Low N/P Ratio Lithium Metal Batteries” Advanced Sustainable Systems, 7(10), 2300231.
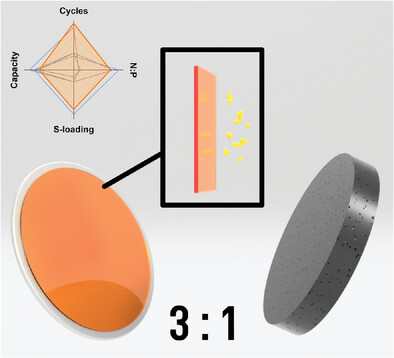
Lithium metal batteries, in particular lithium–sulfur chemistries, hold great promise in energy storage from potentially increased gravimetric storage density and diminished reliance on transition metals, lowering resource demand and hence overall unit cost. However, these cells can have their feasibility improved to a greater extent by lowering the demand for lithium within their construction and reducing the polysulfide shuttling effect. Rising lithium costs and a lack of recycling options indicate the use of excess lithium to mitigate cycling stability issues is sub-optimal. Herein, the direct casting of a lithophilic, superglassy, nanoporous PTMSP polymer separator directly onto the lithium anode is described. PTMSP’s bi-modal, sub-angstrom pore size distribution results in selective rejection of polysulfide species, while its high fractional free volume acts as a stabilizing matrix for deposited lithium as well as possessing a high ionic conductivity of 8.8 × 10-4 S cm−1. The coated anodes exhibit 5.7 times more dense lithium over controls, translating to improved cycling performance due to increased capacity retention and improved lithium utilization at low (< 3) N/P ratios for extended cycle life (> 250 cycles), at practical sulfur loadings (4 mg cm−2). These developments are promising steps for more widespread adoption of lithium sulfur batteries and other metallic lithium systems.
Sue Lyn Tan, Sally El Meragawi, Mainak Majumder, and Ee Von Lau. “Superhydrophilic and underwater superoleophobic Graphene oxide-Phytic acid membranes for efficient separation of oil-in-water emulsions.” Separation and Purification Technology 314 (2023): 123544.
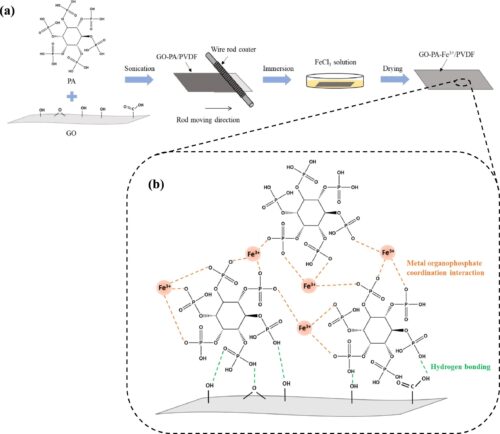
The increase in oily wastewater generation from industrial effluents and accidental oil spills has become a severe environmental concern today. Recently, tailored wettability materials have received extensive research interest due to their potential applications in remediation of oily wastewater. Herein, a superhydrophilic and underwater superoleophobic membrane was synthesised by a two-step approach: surface coating of graphene oxide-phytic acid (GO-PA) mixture on a polyvinylidene fluoride (PVDF) support membrane followed by Fe3+ deposition via metal-organophosphate coordination interaction. The optimised GO-PA-Fe3+/PVDF membrane possessed a water contact angle of 1.4° and an underwater oil contact angle of 163.4°. In the separation of surfactant-stabilised sub-micron sized oil-in-water emulsions (of 0.01 – 1 µm) at 0.4 bar, a permeation flux of up to 43.7 LMH/bar and separation efficiency of > 99.5% were achieved with the synthesised GO-PA-Fe3+/PVDF. This permeation flux was also observed to be six times higher than pristine GO/PVDF. The robust membrane exhibited excellent recyclability with a separation efficiency of > 99% and permeation flux of 38.2 – 41.8 LMH/bar for ten cycles. The research establishes a promising avenue towards tuning Graphene-oxide membranes for treatment of sub-micron sized oily wastewater, while maintaining a high permeation flux.
Sally El Meragawi, Dilusha Cooray, and Mainak Majumder. “Improvement of the chlorine resistance of graphene oxide membranes through siloxane cross-linking.” Separation Science and Technology 58, no. 6 (2023): 1089-1098.
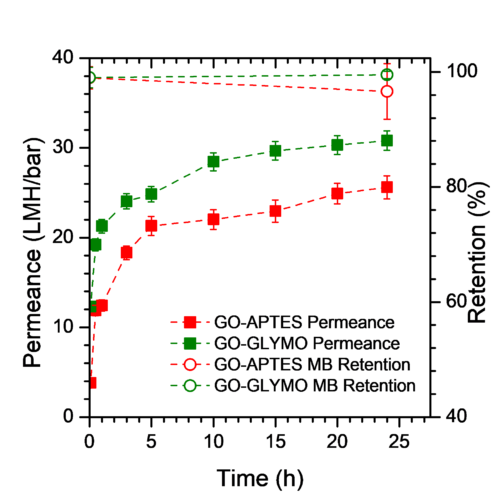
Nanofiltration membranes exhibit performance deterioration when exposed to highly concentrated chlorinated environments. This is a major challenge of nanofiltration process as chlorine-based chemicals are prevalent within industry for the treatment of organic fouling; thus, making membrane chlorine sensitivity detrimental to sustainable operation. Graphene oxide has an inherent chlorine sensitivity and degrades under exposure to high concentration of chlorine. This work describes a novel nanofiltration membrane fabrication strategy in which Polyethyleneimine and siloxane crosslinking chemistries were used to protect the carboxylic, hydroxyl, and epoxy functional groups of GO nanosheets from the attack of free chlorine. These chemistries were used to improve the chlorine-stability of the GO nanosheets. Polyethyleneimine (PEI) reduces GO and cross-links with oxygenated functional groups preventing their further reaction with chlorine. This chemistry was compared to the chlorine-tolerant siloxane cross-linkers which react with GO’s functional groups to decrease reaction sites. The use of (3-Aminopropyl)triethoxysilane (APTES) and (3-Glycidyloxypropyl)trimethoxysilane (GLYMO) cross-linkers resulted in stable retentions that varied by less than 0.5% following 1,000 ppm chlorine exposure for 1 h. Additionally, GLYMO functionalisation of the GO membrane exhibited exceptional chlorine resistance maintaining a methyl blue retention of 98.0 ± 2.15% following 1 h of exposure 30,000 ppm of chlorine.
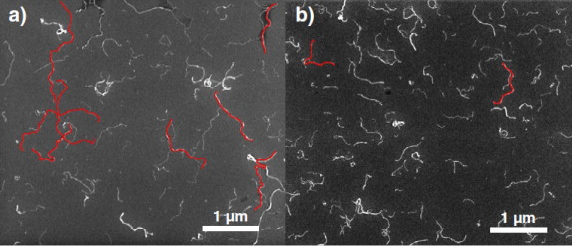
Joynul Abedin, Md, Paul van der Schoot, Gil Garnier, and Mainak Majumder. “Nematic to Cholesteric Transformation in the Cellulose Nanocrystal Droplet Phase.” Langmuir 39, no. 17 (2023): 6142-6150.
Nucleation, growth, and transformation of chirality in nanomaterial systems is a growing research topic with broad interest in tunable and configurable chiroptical materials. Similar to other one-dimensional nanomaterials, cellulose nanocrystals (CNCs), which are nanorods of naturally abundant biopolymer cellulose, display chiral or cholesteric liquid crystal (LC) phases in the form of tactoids. However, the nucleation and growth of the cholesteric CNC tactoids to equilibrium chiral structures and their morphological transformations are yet to be critically assessed. We noticed that the onset of liquid crystal formation in CNC suspensions is characterized by the nucleation of a nematic tactoid that grows in volume and spontaneously transforms into a cholesteric tactoid. The cholesteric tactoids merge with the neighboring tactoids to form bulk cholesteric mesophases with various configurational palettes. We applied scaling laws from the energy functional theory and found suitable agreement with the morphological transformation of the tactoid droplets monitored for their fine structure and orientation by quantitative polarized light imaging.
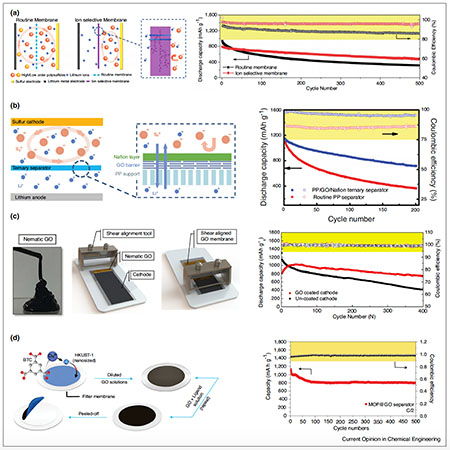
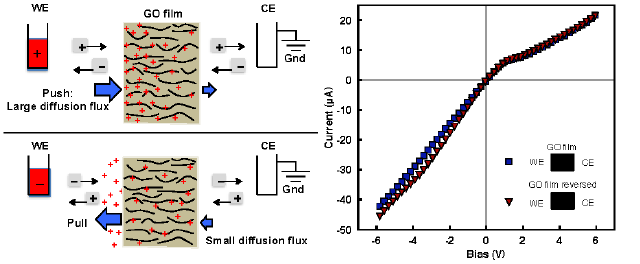
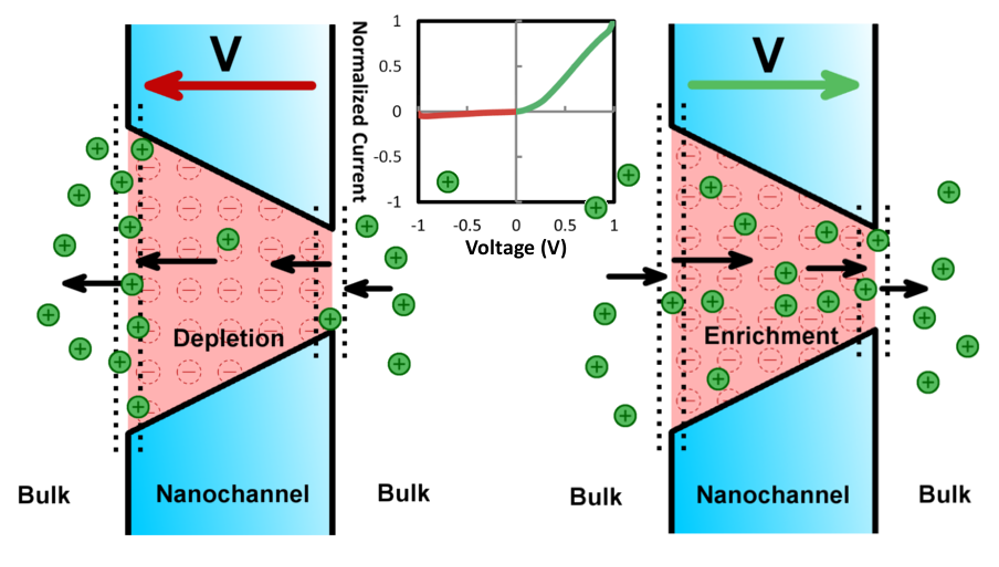
Sally El Meragawi, Manas Ranjan Panda, Petar Jovanović, and Mainak Majumder. “Current challenges and approaches for energy-efficient ion-selective two-dimensional graphene-based channels.” Current Opinion in Chemical Engineering 39 (2023): 100894.
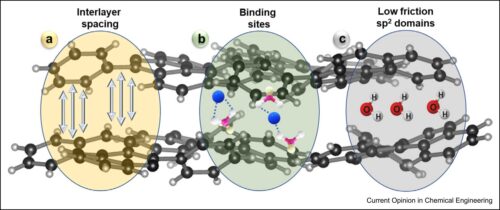
Atomically thin two-dimensional (2D) materials are highly regarded for contemporary separation challenges such as desalination, especially in designing membranes with the potential to overcome the classical permeance-selectivity trade-off challenge, however, critical challenges remain. Graphene oxide (GO)-based membranes have low retention for monovalent ions as instability in aqueous media has yet to be adequately resolved and impacts their widespread application. Beyond GO, other 2D materials such as MoS2 have promising selectivity and permeance properties but are severely hindered by their ability to be scalably produced. By taking inspiration from biological ion channels, a design philosophy can be developed realizing efficient ion selectivity through introducing engineered nanochannel chemistry. Development of 2D material membranes for complex ion separation applications requires looking beyond simple steric effects for discriminating between ions and toward favorable chemical environments for enhancing or hindering ion transport.
Ehsan Ghasemiestahbanati, Young Hee Yoon, Ryan P. Lively, Mahdokht Shaibani, Mainak Majumder, and Matthew R. Hill. “A triple-functional carbon molecular sieve (CMS) that addresses the performance trilemma in practical lithium sulfur batteries.” Carbon 203 (2023): 856-864.
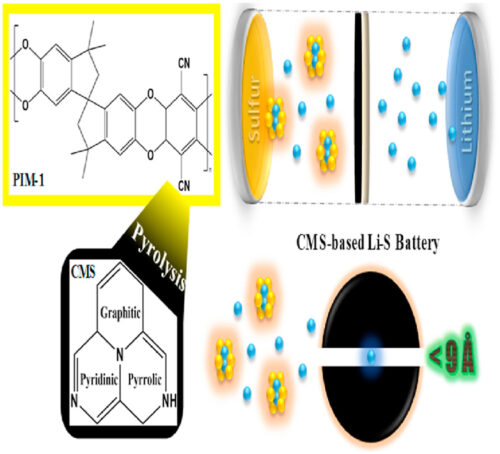
Lithium-sulfur batteries offer an attractive energy storage alternative independent of critical minerals such as cobalt or nickel. However, their inadequate stability under long term cycling, and storage capacity on a volumetric basis hampers their uptake. These limitations can be addressed by seeking to control the formation and crossover of polysulfides, oligomers generated through reaction of solvated sulfur moieties with the lithium anode, and by seeking to restrict the consumption of active materials. Here, we report the development of a triple-functional carbon molecular sieve (CMS) interlayer. The microporous, polar, and conductive structure of the CMS provides physisorption, chemisorption, and reactivation capabilities concurrently. Therefore, the soluble polysulfides can be trapped, suppressed from shuttling, and reutilized to not only improve the kinetics of the redox reaction but also hinder the loss of active materials. As a result, our CMS-based Li–S batteries deliver combined exceptional gravimetric (1282 mAh g−1) and areal (7.05 mAh cm−2) capacities at 0.1 C rate as well as cycling stability up to 1000 cycles at 0.2 C rate over 9 months.
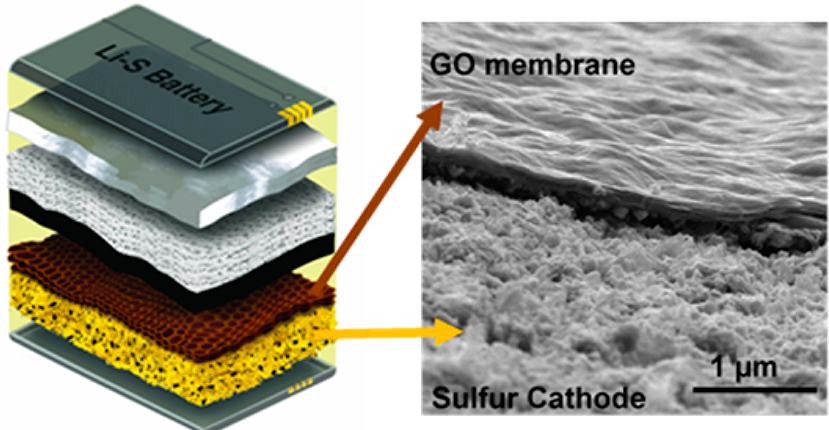
Petar Jovanović, Mahdokht Shaibani, Joynul Abedin, Cara M. Doherty, Durga Acharya, Tanesh Gamot, Anthony F. Hollenkamp, Matthew R. Hill, and Mainak Majumder. “Mimicking a cell plasma membrane to regulate dynamic polysulfide chemistry for a practical lithium-sulfur battery.” Cell Reports Physical Science 3, no. 12 (2022).
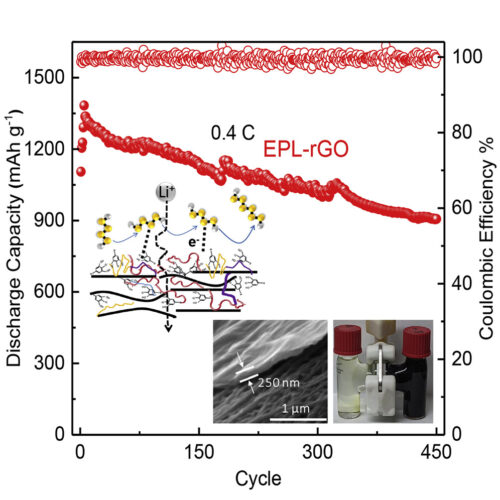
Lithium-sulfur batteries are compelling candidates for overcoming the resource and sustainability limitations of current batteries. Regulating complex polysulfide chemistry is a critical challenge in achieving a practical lithium-sulfur battery with high cycle life and minimal electrolyte weight. Drawing inspiration from cell biology, here we propose the concept of bespoke membranes for making practical lithium-sulfur batteries. The membrane devised herein utilizes conductive reduced graphene oxide as a brick-like framework, with an elastic polymer liquid—rich in ion hopping and lithiophilic sites—as the mortar. The membrane mimics cell plasma membranes by integrating rapid and permselective Li+ channels alongside catalytic electrochemical reactions. Employing our reactive permselective membranes, we attain areal capacities of 4.8–8.1 mAh cm−2 with 450 stable cycles in coin cells and 202 Wh kg−1 with over 100 stable cycles in pouch cells. This behavior is achieved with efficient electrolyte/capacity ratios (4.9–5.3 μL mAh−1).
Yingyi Huang, Mahdokht Shaibani, Md Joynul Abedin, David Joram Mendoza, Zhou Xu, Tanesh Dinesh Gamot, Mahamarakkalage Chrishani Dilusha Cooray et al. “Sulfur Cathodes with Self‐Organized Cellulose Nanofibers in Stable Ah‐Level,> 300 Wh kg− 1 Lithium–Sulfur Cells.” Advanced Energy Materials 12, no. 45 (2022): 2202474.
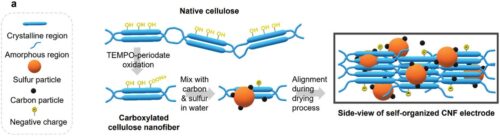
The realization of lithium–sulfur (Li–S) batteries as an energy storage technology depends on unlocking practical performance at commercially relevant pouch cell scales. Typically, the heterogeneous and porous nature of large scale, high sulfur loading Li–S batteries require increased electrolyte levels and impede electronic conductivity. Improved cathode structures offer a pathway to strong performance at large battery scales. Here, the successful development of a new cathode using highly-carboxylated and negatively surface charged cellulose nanofibers as a backbone that addresses these issues and delivers an ordered, dense architecture whilst maintaining long term cycle life, is reported. Taken together this leads to an Ah-level pouch cell with a peak capacity above 1200 mAh g−1 and an areal capacity of around 15 mAh cm−2, which achieves a high gravimetric energy density of up to 330 Wh kg−1 and volumetric energy density of 480 Wh L−1. The cell is used to power a drone for 10 min, demonstrating the ability of this discovery to be translated at practical scales.
Wanqing Chen, Manas Ranjan Panda, Meysam Sharifzadeh Mirsherkaloo, Kourosh Kalantar-Zadeh, and Mainak Majumder. “Photochemically engineered ultra-stable 1T MoS 2 by flow synthesis.” Chemical Communications 58, no. 85 (2022): 11929-11932.
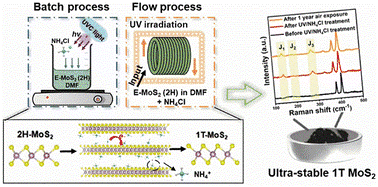
A novel synthesis method for stable conversion of 2H to 1T MoS2 is developed by photoirradiation of ammonium intercalated 2H-MoS2. The synthesized 1T phase in the final product showed excellent long-term stability, and orders of magnitude improvement in electrical conductivity. The denser active sites in the synthesized material contributed to an enhanced HER activity. The methodology can be translated to a continuous flow process paving the way for the large-scale production of 1T MoS2.
Manas Ranjan Panda, Supriya Sau, Rashmi Gangwar, Dhanshree Pandey, Divyamahalakshmi Muthuraj, Wanqing Chen, Aparna Chakrabarti, Arup Banerjee, Archna Sagdeo, Qiaoliang Bao, Mainak Majumder, and Sagar Mitra “An Excellent and Fast Anodes for Lithium-Ion Batteries Based on the 1T′-MoTe2 Phase Material”. ACS Appl. Energy Mater. 2022, 5, 8, 9625–9640
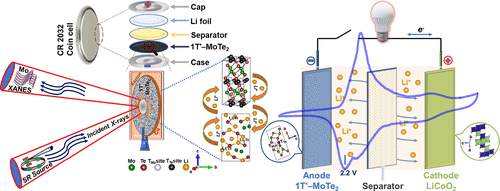
Fast charging battery materials are of incredible interest to the industry as well as in academia. To enhance the fast charging capabilities of batteries, anode materials must have fast Li-ion diffusion and reaction kinetics. The inherent high electronic conductivity and volumetric energy density of semimetallic 1T′-MoTe2 are advantageous as a high-rate anode material for lithium-ion batteries (LIBs). The high mass density of MoTe2 helps to decrease the electrode thickness, thus requiring less electrolyte infiltration favoring a reduction in the auxiliary material and electrolyte costs and indirectly increasing the energy density of the cell. Here, a pristine 1T′-MoTe2 material prepared by a facile and efficient solid-state synthesis route without any addition of carbonaceous additives or surface modifications delivered an initial specific capacity of 538 mAh g–1 with a capacity retention of 92% at 1 A g–1 along with a Coulombic efficiency of 99% over 200 cycles. Ex situ X-ray absorption near-edge structure (XANES) was performed to elucidate the lithium storage mechanism of the 1T′-MoTe2 anode, which was further complemented by lithiation/delithiation calculations performed using density functional theory. Furthermore, the 1T′-MoTe2//LiCoO2 full cell exhibited a reversible specific capacity of 388 mAh g–1 at 100 mA g–1 with a Coulombic efficiency of 96% over 100 cycles, which indicates its potential in fast charging battery cells.
Wanqing Chen, Meysam Sharifzadeh Mirshekarloo, Sally El Meragawi, Geosmin Turpin, Rowan Pilkington, Anastasios Polyzos, Mainak Majumder, “Controlled Nanopore Formation in Graphene/Graphene Oxide Nanosheets: Implication for Water Transport”. ACS Appl. Nano Mater. 2022, 5, 3, 3811–3823
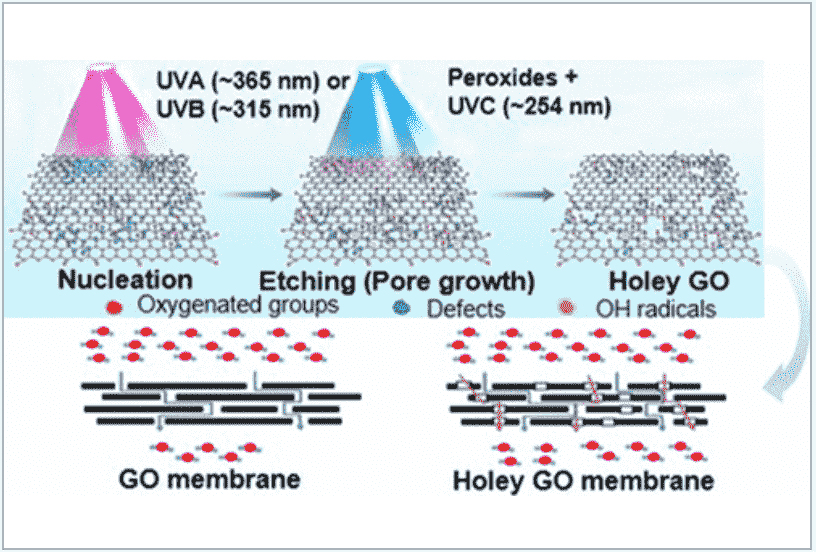
Two-dimensional (2D) sheets of graphene/graphene oxide are the building blocks of a wide range of material architectures with strong application potential in energy storage and harvesting, and environmental remediation. A consistent issue with continuous 2D sheets, especially when hundreds of such 2D sheets are stacked tightly to form films and electrodes, is their low mass transport characteristics through the assembled structure. To overcome this problem, we report a sequential, two-step photochemical technique comprising nucleation of defects on 2D nanosheets of graphene/graphene oxide by long-wavelength (UVA/UVB) irradiation, followed by the growth of nanopores in H2O2-based etching triggered by short-wavelength (UVC) irradiation. We demonstrate our ability to tailor the size (10–100 nm) and level of porosity (16–60%) in holey graphene oxide (h-GO). To test the holey GO we synthesized, we produced the nanofiltration membranes using h-GO with different pore sizes. Membranes made from hGO nanosheets with ∼60 nm pores exhibited up to a 3.7-fold increase in water permeance and an ∼10% increase in selectivity compared to those produced by pristine GO. We attribute this unusual behavior to the presence of water transport highways (the nanopores) and a smaller interlayer distance of the hGO sheets arising from a complex balance in hydroxylation and deoxygenation reactions during the photochemical process. We demonstrated successful transition of the method to a flow-based synthesis approach with highly enhanced production rates (∼188 mg/h, an about 30-fold increase over the batch process), thereby accelerating sustainable and automated manufacturing of perforated graphene materials and their adoption in industrial uses.
Ehsan Ghasemiestahbanati, Mahdokht Shaibani, Kristina Konstas, Barun K. Chakrabarti, C. T. John Low, Mainak Majumder, Matthew R. Hill, “Charge Carrier Molecular Sieve (CCMS) Membranes with Anti-aging Effect for Long-Life Vanadium Redox Flow Batteries”. ACS Appl. Energy Mater. 2022
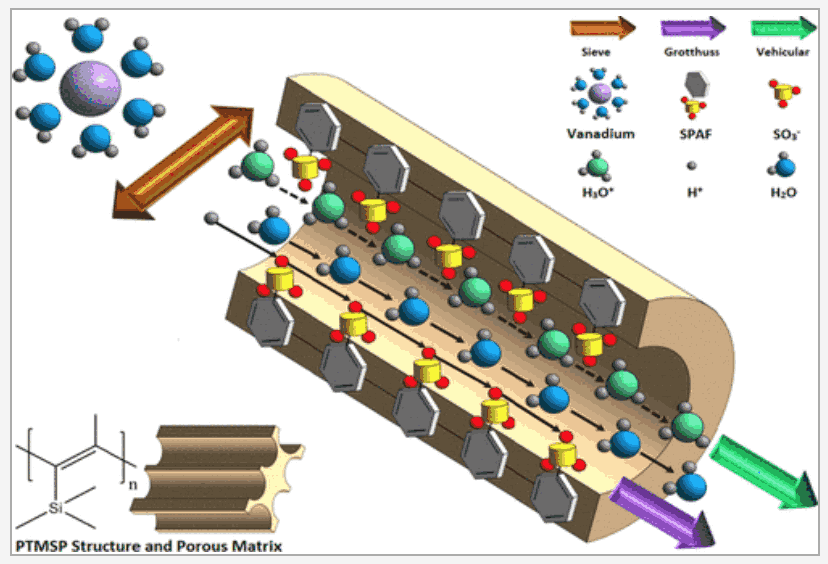
Vanadium crossover hinders widespread commercial adoption of vanadium redox flow batteries (VRFBs). Superglassy polymers have the potential to offer high selectivity needed to control the crossover but as yet do not possess the requisite proton conductivity and stability. Here, we explore nanocomposite separators that can improve this selectivity. We report a dual-function charge carrier molecular sieve (CCMS) membrane, consisting of a high free volume microporous glassy polymer, poly[1-(trimethylsilyl)-1-propyne] (PTMSP)/sulfonated PAF (PAF-1-SO3H), that effectively hinders the migration of hydrated vanadium ions. Furthermore, ideally placed PAF-1-SO3H pores not only proved excellent for developing proton conductive channels but also suppressed physical aging within the separator. Experiments then linked this to an increased battery cycle life. As a consequence of achieving higher and more stable VRFB performance compared to benchmarked Nafion (Coulombic efficiencies of 97 vs 87% and capacity retention values of 85 vs 58% at a current density of 60 mA cm–2, respectively), our integrated design heralds a class of stable separators for hydrogen-based energy technologies.
Ehsan Ghasemiestahbanati, Areeb Shehzad, Kristina Konstas, Caitlin J. Setter, Luke A. O’Dell, Mahdokht Shaibani, Mainak Majumder, Matthew R. Hill, “Exceptional lithium diffusion through porous aromatic framework (PAF) interlayers delivers high capacity and long-life lithium–sulfur batteries
”. J. Mater. Chem. A, 2022,10, 902-911
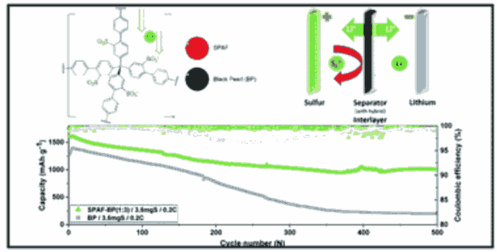
Lithium–Sulfur (Li–S) batteries offer potential for significant energy density storage gains in concert with the use of sustainable electrode materials. Their cycling stability and slow charging kinetics need significant improvement for deployment in practical settings. Consequently, facilitated transport within the cell is critical for promoting lithium transport whilst simultaneously retarding the movement of polysulfides, which limit the capacity and stability. We have developed a nanoporous interlayer with an ideal pore architecture and surface chemistry that overcomes these challenges. Porous Aromatic Frameworks (PAFs) have uniform 13 Å pores and exceptional internal surface areas that can be readily chemically functionalized. Appropriate sulfonation levels (SPAF) within these pores can deliver exceptional lithium ion transport rates, concomitant with the repulsion of unwanted polysulfide moieties. This combination of properties leads to outstanding capacity retention, above 1000 mA h g−1 after 500 cycles at practical charge rates. Our high capacity and cyclable battery design is also supported by high coulombic efficiency (av. >99.5%) and sulfur utilization (∼96%) as well as excellent lithium anode protection. These remarkable properties have been methodically explored with a suite of analytical techniques that link the battery performance to the fundamental physicochemical properties of the SPAF hybrid interlayer.
Yingyi Huang, Mahdokht Shaibani, Tanesh D. Gamot, Mingchao Wang, Petar Jovanović, M. C. Dilusha Cooray, Meysam Sharifzadeh Mirshekarloo, Roger J. Mulder, Nikhil V. Medhekar, Matthew R. Hill, Mainak Majumder, “A saccharide-based binder for efficient polysulfide regulations in Li-S batteries
”. Nat. Comm., vol. 12, (2021)
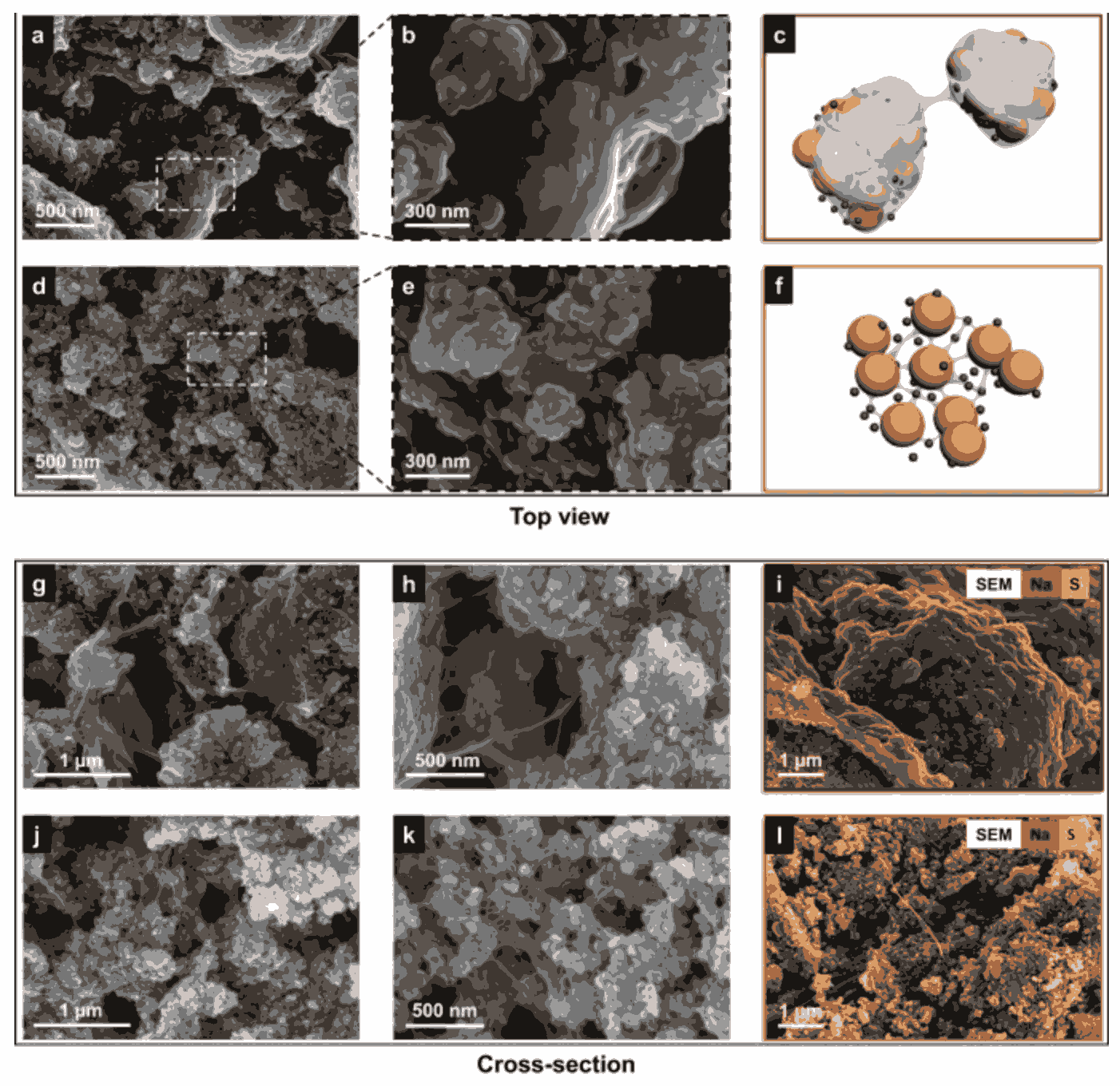
The viability of lithium-sulfur batteries as an energy storage technology depends on unlocking long-term cycle stability. Most instability stems from the release and transport of polysulfides from the cathode, which causes mossy growth on the lithium anode, leading to continuous consumption of electrolyte. Therefore, development of a durable cathode with minimal polysulfide escape is critical. Here, we present a saccharide-based binder system that has a capacity for the regulation of polysulfides due to its reducing properties. Furthermore, the binder promotes the formation of viscoelastic filaments during casting which endows the sulfur cathode with a desirable web-like microstructure. Taken together this leads to 97% sulfur utilisation with a cycle life of 1000 cycles (9 months) and capacity retention (around 700 mAh g−1 after 1000 cycles). A pouch cell prototype with a specific energy of up to 206 Wh kg−1 is produced, demonstrating the promising potential for practical applications.
Sally El Meragawi, Abozar Akbari, Tanesh Gamot, Akshat Tanksale, Mainak Majumder, “High-Performance Nanofiltration Membranes from Polyphenol–Graphene Oxide Liquid Crystals Prepared Using Natural Extract”. ACS Sustain. Chem. Eng. 2021
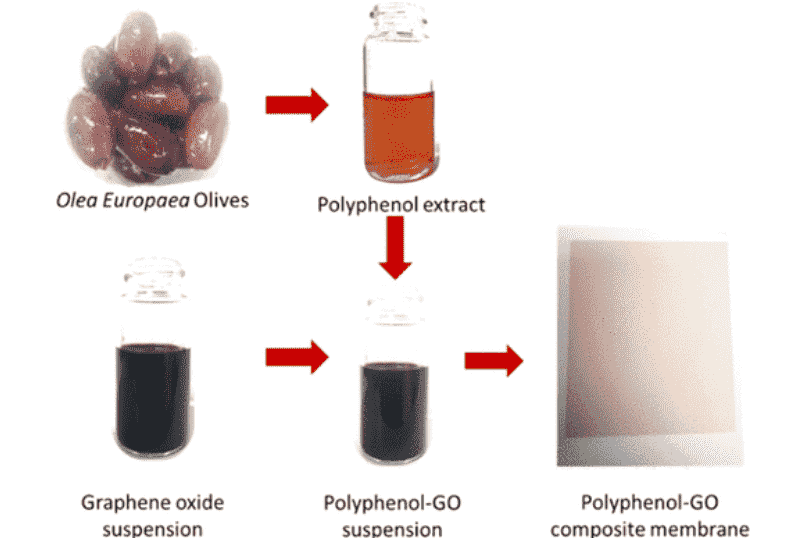
Oxygen functionalities on graphene oxide (GO) nanosheets have a significant role in the performance of GO-based laminar membranes. The influence of these groups on nanochannel spacing, electrostatic repulsion, and transport resistance in aqueous and polar environments is well recognized. In this work, the antioxidative properties of olives, Olea europaea, were exploited to gradually reduce GO and the effects of progressive deoxygenation on the properties GO membranes were systematically monitored. The optimization of the reaction process in this manner enabled the fabrication of an ultrafast membrane with enhanced molecular sieving characteristics. Ultrathin (∼30 nm thick) membranes prepared from liquid crystalline polyphenol–GO dispersions with a water permeance of 60.4 ± 2.8 L·m–2·h–1·bar–1 showed dramatic improvement over GO membranes (water permeance of 10 ± 3.4 L·m–2·h–1·bar–1). This is combined with enhanced molecular sieving characteristics of >90% for probes such as methyl orange with hydrated radii greater than 5.0 Å and stability in crossflow filtration tests wherein Rose Bengal (974 Da) is retained at >90% for 100 h compared to GO, which falls below 50% retention in the same amount of time. This improvement is attributed to the confluence of the loss of oxygen functional groups and cross-linking attachment of polyphenols to the GO nanosheets. The results will facilitate new understanding in the design of novel bio-inspired composite membranes.
Meysam Sharifzadeh Mirshekarloo, M. C. Dilusha Cooray, Petar Jovanović, Christopher D. Easton, Fan Wu, Tanesh D. Gamot, Md. Joynul Abedin, Mehmet Rasit Yuce, Mahdokht Shaibani, and Mainak Majumder, “Liquid-Crystal Mediated Assembly of Iodinated Graphene Oxide for Ultra-Dense Supercapacitors as Safe Power Source for Internet of Things Data Transmission ”. Batteries & Supercaps, 2021, 4, 1175-1185
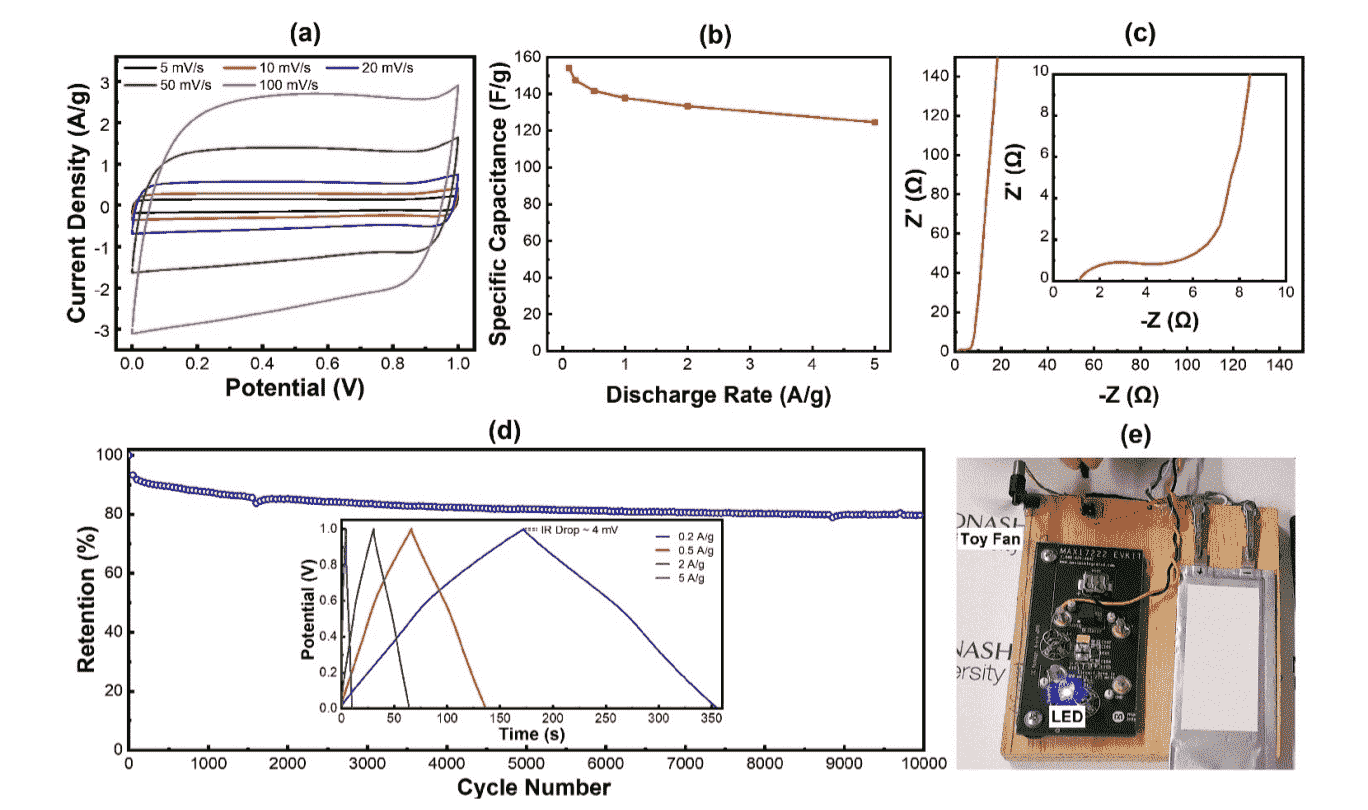
Dense iodinated reduced graphene oxide (rGO) with outstanding electrical and electrochemical properties is produced through a green method comprising liquid crystal‐mediated assembly of polyiodides and GO, and subsequent UV irradiation. The dense rGO electrode (1.46 g/cm3) exhibited a very high volumetric capacitance of 226 F/cm3, a factor of three larger than commercial activated carbon, and amongst the highest reported for graphene. The scalability is demonstrated by the fabrication of supercapacitor pouch cells that realized a volumetric energy density of 0.94 Wh/l, comparable to market products with similar footprint, while advantageously using a safe and green aqueous electrolyte. Our pouch cell powered battery‐less Internet of Things (IoT) sensor nodes and demonstrated sensing and transmission of over 40 temperature and relative humidity data packets. Our work establishes the critical advantages graphene‐based materials have over activated carbons in terms of ease of fabrication, tailorability, and enhanced volumetric energy density to advance the state‐of‐art in supercapacitor device research.
Petar Jovanović, Meysam Sharifzadeh Mirshekarloo, Matthew R. Hill, Anthony F. Hollenkamp, Mainak Majumder, and Mahdokht Shaibani,“ Separator Design Variables and Recommended Characterization Methods for Viable Lithium–Sulfur Batteries ”. Adv. Mater. Technol. 2021, 2001136
The lithium–sulfur (Li–S) battery is at the forefront of technologies that can outperform lithium-ion in at least one index of performance, provided that solutions to poor cycle-life can be devised. One key component of the Li–S battery is the separator, because it holds tremendous promise for improving cycle-life by mitigating the well-known polysulfide shuttle, enabling lean electrolyte configurations, and restricting solid electrolyte interphase growth at the Li-metal anode. However, in response to the advent of the “functional separator” for Li–S batteries, severe misinterpretations of progress have been made due to the often incomplete presentation of the performance
and characterization criteria for these new components. Accordingly, there
is an urgent need to look critically at what has been achieved in Li–S separator research, with the aim of reconciling actual progress against claimed improvements. This review advocates the best practices for reporting the performance of Li–S separators and proposes guidelines on measurements with respect to key properties. It is believed that the adoption of these measurement practices, testing, and reporting styles will enable more accurate determination of separator performance and properties, which in turn can allow for more meaningful comparisons between various approaches, as well as facilitating the transition of laboratory concepts to practical designs.
Sally El Meragawi, Abozar Akbari, Sebastian Hernandez, Meysam Sharifzadeh Mirshekarloo, Dibakar Bhattacharyya, Akshat Tanksale, and Mainak Majumder,“ Enhanced permselective separation of perfluorooctanoic acid in graphene oxide membranes by a simple PEI modification”. J. Mater. Chem. A, 2020,8, 24800
Perfluoroalkyl compounds of various molecular weights have emerged as a class of persistent contaminants with profound negative impact on human health and the environment. The selectivity and permeance of Graphene Oxide (GO) and amine surface functionalised GO nanofiltration membranes were assessed for the removal of perfluorooctanoic acid (PFOA _400 Da) in concentration ranges relevant to availability in wastewater. GO nanofiltration membranes demonstrated a reasonable efficiency of 74.3% at 50 ppm of PFOA and a water permeance of 10 _ 2.1 L m_2 h_1 bar_1. By functionalising the top surface with polyethyleneimine (PEI), the GO membrane underwent reduction and cross-linking reactions. The modified membranes demonstrated improved mechanical stability and an enhanced retention of 96.5% at 50 ppm PFOA and a permeance of 15.9 _ 1.3 L m_2 h_1 bar_1. The electron rich PEI deoxygenates GO leading to a smaller interlayer spacing, but also increases the surface hydrophilicity—the combination of both these properties leads to increased PFOA retention by steric hindrance and enhanced water permeation. As steric hindrance effects were the dominant mechanism of retention, the GO-PEI membranes operated more effectively (retention >90%) in a much wider range of concentrations (100 ppb to 100 ppm), compared to the GO membrane. Our research demonstrates that strategic surface-modification techniques can tailor the effectiveness of GO-based loose nanofiltration membranes for the retention of emerging contaminants while maintaining lower osmotic pressure effects through salt retention minimisation.
Md. Joynul Abedin, Titon Barua, Mahdokht Shaibani, Mainak Majumder,“A High Throughput and Unbiased Machine Learning Approach for Classification of Graphene Dispersions”. Adv. Sci. 2020, 7, 2001600
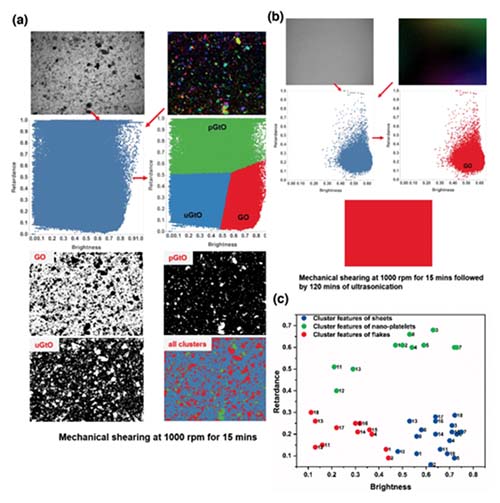
Significant research to define and standardize terminologies for describing stacks of atomic layers in bulk graphene materials has been undertaken. Most methods to measure the stacking characteristics are time consuming and are not suited for obtaining information by directly imaging dispersions. Conventional optical microscopy has difficulty in identifying the size and thickness of a few layers of graphene stacks due to their low photon absorption capacity. Utilizing a contrast based on anisotropic refractive index in 2D materials, it is shown that localized thickness‐specific information can be captured in birefringence images of graphene dispersions. Coupling pixel‐by‐pixel information from brightfield and birefringence images and using unsupervised statistical learning algorithms, three unique data clusters representing flakes (unexfoliated), nanoplatelets (partially exfoliated), and 2D sheets (well‐exfoliated) species in various laboratory‐based and commercial dispersions of graphene and graphene oxide are identified. The high‐throughput, multitasking capability of the approach to classify stacking at sub‐nanometer to micrometer scale and measure the size, thickness, and concentration of exfoliated‐species in generic dispersions of graphene/graphene oxide are demonstrated. The method, at its current stage, requires less than half an hour to quantitatively assess one sample of graphene/graphene oxide dispersion
Petar Jovanović, Mahdokht Shaibani, Meysam Sharifzadeh Mirshekarloo, Yingyi Huang, Kristina Konstas, Areeb Shehzad, Matthew R. Hill, Mainak Majumder,“ Designer Self-Assembled Polyelectrolyte Complex Nanoparticle Membrane for a Stable Lithium–Sulfur Battery at Lean Electrolyte Conditions”. ACS Appl. Energy Mater. 2020, 3, 8, 7908
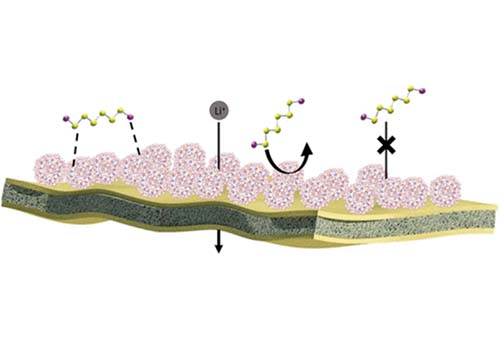
Lithium–sulfur batteries can displace lithium-ion batteries owing to their superior theoretical capacity and specific energy density. Presently, however, high specific capacities do not translate to high specific energies, mainly because of the electrolyte excess, which does not meet the required “lean electrolyte” condition. We introduce a separator that requires a minimal amount of electrolyte, 4.5 μL mg–1, for successful cycling of practical sulfur cathodes. Taking advantage of the self-assembly chemistry of polyelectrolyte complexation, we synthesized a tailored porous nanoparticle, which because of its amphiphilicity, is able to form a submicron coating on the low-surface energy Celgard separator by dip-coating. The tuned pore-size in the range of 1.5–2 nm, abundance of functional groups, and unprecedented adsorption capacity toward LiPS allows the polyelectrolyte complex nanoparticle decorated (PPX) separator to function as an efficient LiPS modulator, while uniquely maintaining lean electrolyte conditions and excellent transport properties. The PPX separator enabled a cell with capacity of 1348 mAh g–1 (5.12 mAh cm–2) at 0.2 C. Achieving the challenging trade-off between high capacity and lean electrolyte, we were able to attain high energy density in a pouch cell prototype with an initial capacity of 1218 mAh g–1 and an energy density of 250 Wh kg–1.
Manas Ranjan Panda, Rashmi Gangwar, Divyamahalakshmi Muthuraj, Supriya Sau, Dhanshree Pandey, Arup Banerjee, Aparna Chakrabarti, Archna Sagdeo, Matthew Weyland, Mainak Majumder, Qiaoliang Bao, Sagar Mitra,“ High Performance Lithium‐Ion Batteries Using Layered 2H‐MoTe2 as Anode”. Small. 2020, 16, 2002669
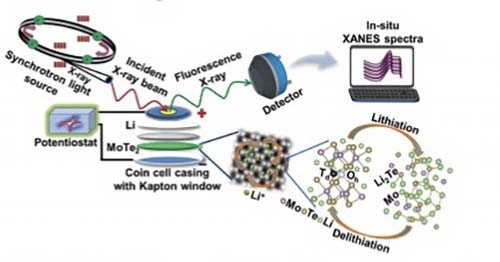
The major challenges faced by candidate electrode materials in lithium‐ion batteries (LIBs) include their low electronic and ionic conductivities. 2D van der Waals materials with good electronic conductivity and weak interlayer interaction have been intensively studied in the electrochemical processes involving ion migrations. In particular, molybdenum ditelluride (MoTe2) has emerged as a new material for energy storage applications. Though 2H‐MoTe2 with hexagonal semiconducting phase is expected to facilitate more efficient ion insertion/deinsertion than the monoclinic semi‐metallic phase, its application as an anode in LIB has been elusive. Here, 2H‐MoTe2, prepared by a solid‐state synthesis route, has been employed as an efficient anode with remarkable Li+ storage capacity. The as‐prepared 2H‐MoTe2 electrodes exhibit an initial specific capacity of 432 mAh g−1 and retain a high reversible specific capacity of 291 mAh g−1 after 260 cycles at 1.0 A g−1. Further, a full‐cell prototype is demonstrated by using 2H‐MoTe2 anode with lithium cobalt oxide cathode, showing a high energy density of 454 Wh kg−1 (based on the MoTe2 mass) and capacity retention of 80% over 100 cycles. Synchrotron‐based in situ X‐ray absorption near‐edge structures have revealed the unique lithium reaction pathway and storage mechanism, which is supported by density functional theory based calculations.
Pritishma Lakhe, Devon L. Kulhanek , Xiaofei Zhao , Maria I. Papadaki , Mainak Majumder, Micah J. Green,“ Graphene Oxide Synthesis: Reaction Calorimetry and Safety”. Ind. Eng. Chem. Res. 2020, 59, 19, 9004–9014,
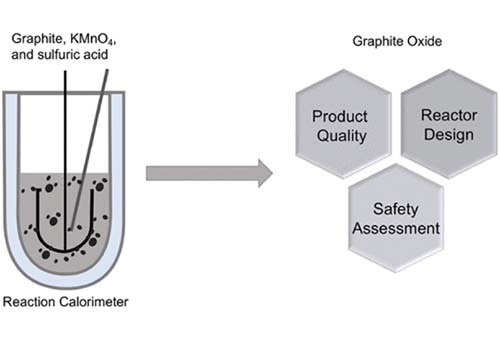
While several studies have been published to optimize the oxidation–exfoliation process of modified Hummers’ method to make graphite oxide (GO), relatively few studies have explored the effects of operating conditions on the final GO product or process safety concerns with the GO synthesis process. In this study, reaction calorimetry is used to determine the heat of solution and oxidation reaction for a modified Hummers’ method as a function of reactor processing parameters. We find that the heat of reaction increases when graphite is soaked in sulfuric acid for an extended time compared to an oxidation process without extended soaking of graphite in acid. GO synthesized with acid-soaked parent material has more surface functional groups, and the heat of the oxidation reaction decreases with increasing stirring rate. In contrast, GO synthesized with non-acid-soaked parent material has more edge functionalized groups and the heat of reaction does not vary with stirring speed. The study shows the heats of solution and reaction are high enough to reach the reported unstable temperature of Mn2O7; however, the amount of Mn2O7 generated in a typical modified Hummers’ method is dilute enough to avoid a violent reaction at 55 °C.
Nicolas Eshraghi, Loris Berardo, Audrey Schrijnemakers, Vincent Delaval, Mahdokht Shaibani, Mainak Majumder, Rudi Cloots, Bénédicte Vertruyen, Frédéric Boschini, Abdelfattah Mahmoud,“ Recovery of Nano-Structured Silicon from End-Of-Life Photovoltaic Wafers with Value-Added Applications in Lithium-Ion Battery”. ACS Sustainable Chem. Eng. 2020, 8, 15, 5868-5879.
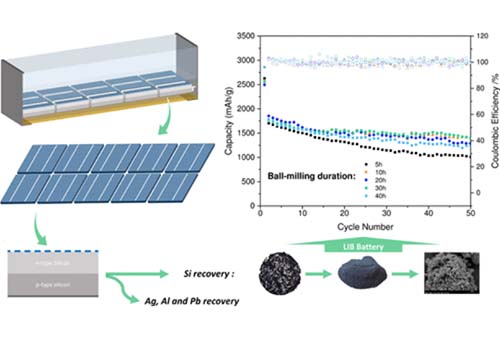
Millions of residential and industrial solar panels installed in the late 1980s and early 1990s are approaching the end of their life, resulting in the drastic accumulation of a potential source of environmental pollution—given the presence of hazardous materials, such as lead. The foreseen crisis, however, can be turned into a great opportunity by value-added recovery of precious solar-grade silicon (Si) to the highly desired nanostructured silicon for lithium-ion batteries (LIBs). Herein, we demonstrate a potential end-of-life management option for photovoltaic (PV) panels, representing a step toward producing greener and more energy-efficient Si for batteries. We show that leaching the recovered silicon wafers in critically tuned alkali-acid leaching baths effectively removes the major impurities: lead (Pb), silver (Ag), and aluminum (Al). The ultrapure Si is then nanosized via industrially scalable milling routes to meet the requirements of expansion-tolerant Si anodes for LIBs delivering capacities as high as 1400 mAh g–1.
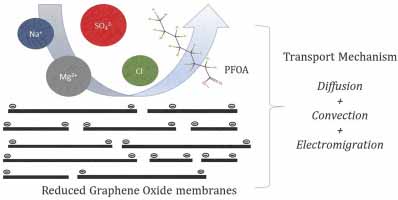
Ashish Aher , Trisha Nickerson, Clair Jordan, Fox Thorpe, Evan Hatakeyama, Lindell Ormsbee, Mainak Majumder, Dibakar Bhattacharyya,“ Ion and Organic Transport in Graphene Oxide Membranes: Model Development to Difficult Water Remediation Applications”. J. Membr. Sci. 604, 2020, 118024.
The role of steric hindrance and charge interactions in governing ionic transport through reduced graphene oxide (rGO) and commercial (DOW-Filmtec NF270) membranes was elucidated by a comprehensive study of experimental and established mathematical analysis based on Nernst-Planck equation. A charge-dominated salt exclusion mechanism was observed for the rGO membranes, which exhibited retention from low (7%) to moderate (70%) extent depending on the nature of ions (5mM). Swelling of GO (1.2 nm interlayer distance) in water beyond the hydrated diameter of ions was attributed as a primary cause for lowering steric hindrance effects. The influence of parameters affecting charge interactions, such as pH and ionic strength, on the extent of salt rejection was modeled. The potential impact of the membrane’s charge density, GO loading and interlayer spacing on salt retention was quantified by performing sensitivity analyses. For a high TDS produced water sample, the rGO membranes partially retained divalent cations (Ca:13%) and exhibited high dissolved oil rejection. The membranes were found to be suitable for the treatment of high TDS water with the goal of selectively removing organic impurities, and thus minimizing the impact of osmotic pressure effect. Performance of the membranes was also investigated for retention of water remediation related organic anions, using perfluoro octanoic (PFOA) acid as a model compound. rGO membranes exhibited a charge-dominated exclusion mechanism for retention (90%) of PFOA (1ppm).
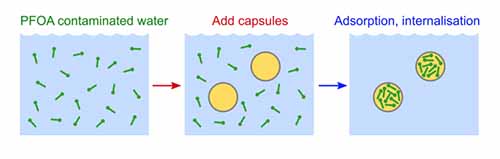
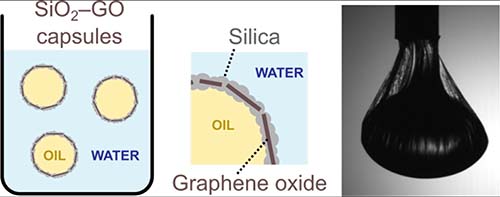
Muthana Ali, Shane P. Meaney, Phillip Holt, Mainak Majumder, Rico F. Tabor,“ Capture of Perfluorooctanoic Acid using Oil Filled Graphene Oxide–Silica Hybrid Capsules”. Environ. Sci. Technol., 2020, 54, 6, 3549-3558.
Fluorinated hydrocarbon (FHC) contamination has attracted global attention recently, due to persistence within the environment and ecosystems of many types of FHC. The surfactant perfluorooctanic acid (PFOA) is particularly commonly found in contaminated sites, and thus needs urgent action to be removed from the environment. In this study, water dispersible hybrid capsules were successfully prepared from an oil-in-water emulsion stabilized by graphene oxide and including a silicate precursor, to grow a strong and mesoporous capsule shell surrounding the droplets. These capsules were decorated with amine groups to present a positively charged outer corona that attracts negative PFOA molecules. The aminated capsules were effectively applied as a novel technology to adsorb and sequester PFOA contamination in water. It was confirmed that PFOA removal by the capsules was pH and PFOA concentration dependent, with adsorption efficiencies of >60 mg/g in ideal conditions. PFOA removal kinetics followed using HPLC and LCMS showed that capture of PFOA by the capsules reached a maximum of >99.9% in 2–3 days.
M. Shaibani, M. S. Mirshekarloo, R. Singh, C. D. Easton, M. C. D. Cooray, N. Eshraghi, T. Abendroth, S. Dörfler, H. Althues, S. Kaskel, A. F. Hollenkamp, M. R. Hill, M. Majumder, “Expansion-tolerant architectures for stable cycling of ultrahigh-loading sulfur cathodes in lithium-sulfur batteries”. Sci. Adv. 6, eaay2757 (2020).
Lithium-sulfur batteries can displace lithium-ion by delivering higher specific energy. Presently, however, the superior energy performance fades rapidly when the sulfur electrode is loaded to the required levels—5 to 10 mg cm−2— due to substantial volume change of lithiation/delithiation and the resultant stresses. Inspired by the classical approaches in particle agglomeration theories, we found an approach that places minimum amounts of a high-modulus binder between neighboring particles, leaving increased space for material expansion and ion diffusion. These expansion-tolerant electrodes with loadings up to 15mg cm−2 yield high gravimetric (>1200 mA·hour g−1) and areal (19 mA·hour cm−2) capacities. The cells are stable for more than 200 cycles, unprecedented in such thick cathodes, with Coulombic efficiency above 99%.
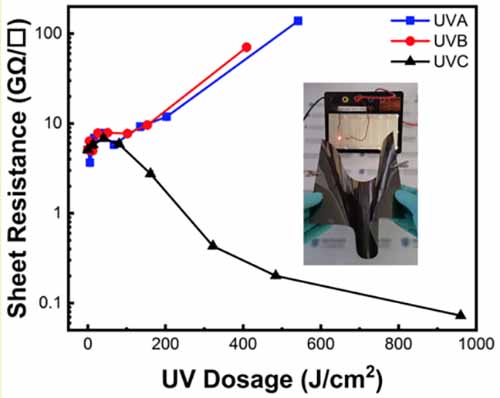
Meysam Sharifzadeh Mirshekarloo, Mahdokht Shaibani, M. C. Dilusha Cooray, Christopher D. Easton, Laure Bourgeois, Sebastian Hernandez, Petar Jovanović, Ludovic F. Dumée, Parama Chakraborty Banerjee, Mainak Majumder,“UV-Assisted Fabrication of Green Quality rGO with Wavelength-Dependant Properties”. ACS Sustainable Chem. Eng. 2020, 8, 2, 1031-1042
Reduction of graphene oxide (GO) has emerged as one of the most feasible and cost-efficient routes to graphene-based materials. While many approaches reliant on the use of chemicals and/or irradiation are known to restore the π-bonds and partially revive the properties of graphene, they suffer from issues such as scalability, waste generation, and adaptability to wide variety of product needs. Herein, a simple, yet versatile solid-state method for tuning the properties of GO by balancing the competing mechanisms of graphitization and defect generation is reported. Such demand cannot be met by the current irradiation-based reduction routes. Healing of π–π bonds and graphitization are found to be promoted by UVC radiation, whereas defect production and amorphisation are distinctly prominent with UVA treatment. The healed rGO films are suitable for production of supercapacitors in commercial-size configurations with competitive volumetric energy densities (rGO electrode capacitance of 194.3 F/cm3 translated to electrode energy density of 27 W h/L), whereas the defective graphene (UVA treated) was found to increase the permeance of GO nanofiltration membranes to fivefold without suppressing its rejection characteristics. It is shown that critical selection of wavelengths of the UV light is key to tune the final properties of rGO for targeted applications.
Sally El Meragawi, Abozar Akbari, Sebastian Hernandez, Akshat Tanksale, Mainak Majumder,“ Efficient Permeance Recovery of Organically Fouled Graphene Oxide Membranes”. ACS Appl. Bio Mater. 2020, 3, 1, 584-592
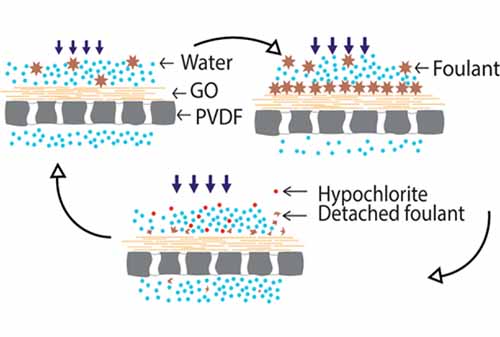
The emergence of facile approaches for the large-scale production of graphene oxide (GO) membranes necessitates a clearer understanding of their potential to foul and, more importantly, strategies for efficient recovery of membrane performance following fouling. Here, we systematically investigated the feasibility of water, ethanol, and hypochlorite as cleaning agents to remove organic foulants over a GO membrane. Among them, 100 ppm hypochlorite solution showed a remarkable ability to remove bovine serum albumin (BSA) and could recover the membrane flux up to 98% after five cycles of BSA filtration and cleaning. The potential of hypochlorite was also demonstrated for permeance recovery during molecular filtration of tannic acid and methyl blue. Scanning electron microscopy, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), and X-ray diffraction (XRD) analyses were used to study the oxidative effects of hypochlorite on the GO membrane, and it was determined that exposure to higher concentrations of hypochlorite (>1000 ppm) degrades the structure of GO membrane and deteriorates the membrane performance after three cycles of cleaning. The studies demonstrate that the use of a modest concentration of hypochlorite is effective in restoring permeance of this class of high flux nanofiltration membranes.
Albert Guirguis, James W. Maina, Lingxue Kong, Luke C. Henderson, Akshita Rana, Lu Hua Li, Mainak Majumder, Ludovic F. Dumee, “Perforation routes towards practical nano-porous graphene and analogous materials engineering”, Carbon,155, 2019, 660
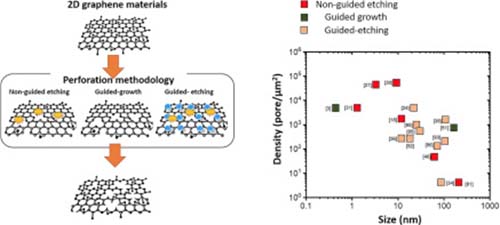
Nano-perforated graphene sheets have emerged as exciting two-dimensional materials for a broad range of scientific and commercial purposes, due to their modified physicochemical properties as compared to native graphene materials. Nanoporous graphene sheets as a class of two-dimensional materials with thicknesses ranging from sub-nanometre to few tens of nanometres, possess high specific surface areas and porous mesh structures with tuneable porosity levels. These properties lead to high densities of unsaturated carbon edges around the pores, making them attractive candidates for applications such as energy storage, separation, sensing or catalysis. Several perforation methodologies have been reported to sculpt pores across graphene structures via etching or guided growth mechanisms. This review focuses on current and emerging nano-perforation methodologies for the two-dimensional graphene materials, and discusses controllable porosity parameters in terms of physical pore size and surface pore density across 2D materials. The relationship between perforation methodology and the achieved porosity level is also discussed and related to electronic or surface reactivity properties. Suggestions towards perforation methodologies in relation to targeted pore size and density, as well as the current challenges hindering scalability of engineering the nanoporous graphene and other similar two-dimensional materials are also highlighted.
Prashant Agrawal, Prasanna S. Gandhi, Mainak Majumder, and Prasoon Kumar “Insight into the Design and Fabrication of a Leaf-Mimicking Micropump”, Phys. Rev. Applied 12, 2019, 031002
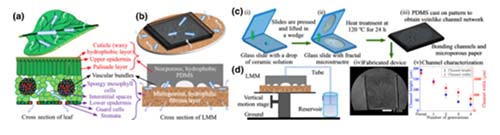
A micropump is the heart of any microfluidic device that finds applications in several lab-on-chip devices. Passive micropumps are highly desirable for this purpose due to their ease of integration, low energy requirements, and simplistic design and operation. The design of a plant leaf serves as natural inspiration for the development of an evaporation-assisted passive micropump. The presence of a branching-channel-like venation pattern ensures water distribution to the spongy mesophyll cells, increasing the surface area for evaporation. However, because of its multiscale design and the complexity of the venation pattern, emulating a leaf’s design is challenging. Apart from the lack of understanding of design parameters that affect fluid flow, manufacturing limitations impede the development of such bioinspired micropumps. Inspired by the multiscale design of the leaf, in this work we propose a passive micropump mimicking the structure of a leaf. Using evaporation and capillary pressure as the pumping mechanism, our leaf-mimicking micropump consists of a microporous membrane integrated with a branched, fractal channel network resembling a leaf’s venation pattern. Our proposed fabrication method is simple, scalable, and inexpensive and uses readily available materials. We demonstrate a significant increase in the fluid flow rate due to the addition of this branched-channel network. We support our experimental observations using an analytical model, wherein we discuss the design parameters that affect the pumping rate. Correspondingly, the performance of these micropumps can be optimized on the basis of intrinsic and extrinsic factors as per the desired applications.
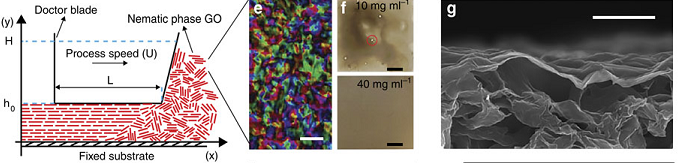
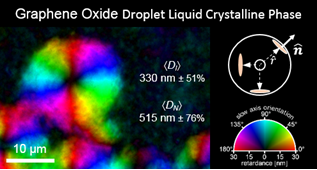
Md. Joynul Abedin, Tanesh D. Gamot, Samuel T. Martin, Muthana Ali, Kazi Imdadul Hassan, Meysam Sharifzadeh Mirshekarloo, Rico F. Tabor, Micah J. Green, Mainak Majumder, “Graphene Oxide Liquid Crystal Domains: Quantification and Role in Tailoring Viscoelastic Behavior”, ACS Nano,2019,13,8,8957-8969
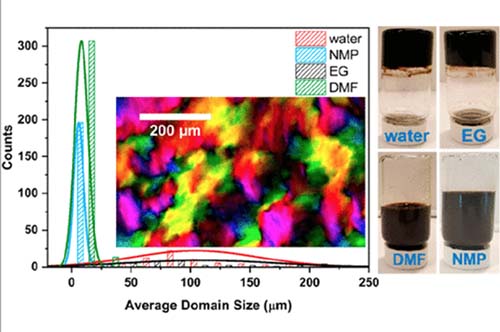
Graphene oxide liquid crystals (GOLCs) were exfoliated in a wide variety of solvents (water, ethylene glycol (EG), N-methyl-2-pyrrolidone (NMP), and dimethylformamide (DMF)) by high-speed shearing of graphite oxide. Quantitative polarized light imaging of the equilibrium nematic phases of the lyotropic GOLCs gives insights into the extent of aggregation and quantifiable textural features such as domain size, d. Large nematic domains >100 μm with a high overall degree of order were obtained in water and ethylene glycol, in contrast to ∼5–50 μm domains in NMP and DMF at comparable volume fractions. Comprehensive rheological studies of these GOLCs indicate that larger domains correlate with higher viscosity and higher elasticity, and scaling analysis shows a power-law dependence of the Ericksen number (Er) with domain size (Er ∝ d3.09). The improved understanding of the relationship between the microstructure and flow properties of GOLCs leads us to an approach of mixed solvent-based GOLCs as a means to tune viscoelastic properties. We demonstrate this approach for the formation of shear-aligned GOLC films for advanced flexible electronic applications such as all-carbon conductive films and thermal heaters
Muthana Ali, Shane P. Meaney, Md. Joynul Abedin, Phillip Holt, Mainak Majumder, Rico F. Tabor, “Graphene oxide–silica hybrid capsules for sustained fragrance release”, J. Colloid Interface Sci., 2019, 552, pp 528-539
Encapsulation of active or valuable cargoes has become one of the most important methods for controlled delivery and release. However, many existing capsule technologies suffer from scalability issues, and capsules from surfactant- or polymer-stabilised emulsions tend to have weak shells or limited stability. Here we present a robust and scalable method for the surfactant-free preparation of silica hybrid capsules templated from Pickering emulsions stabilised by graphene oxide. These capsules are produced using a single step, undemanding formulation process with cheap and scalable precursors. The mechanical and chemical stability provided by the silica shell grown around these droplets is explored using surface pressure measurements and atomic force microscopy, demonstrating that a rigid and robust capsule is produced from higher loadings of silica precursor. In order to demonstrate the utility of these capsules, the sustained release of a fragrance molecule (vanillin) from the capsules is monitored, and compared to release from unencapsulated vanilla oil. It is seen that the capsules retain the fragrance for multiple weeks, offering new pathways for scalable encapsulation systems for the delivery of valuable actives.
Tanesh D. Gamot, Arup R. Bhattacharyya,Tam Sridhar, Alex J. Fulcher, Fiona Beach, Rico F. Tabor, Mainak Majumder “Enhanced Thermal Conductivity of High Internal Phase Emulsions with Ultra-Low Volume Fraction of Graphene Oxide”, : Langmuir 2019, 35, 2738−2746
Thermal conductivity enhancement in a multiphase fluid such as water-in-oil emulsion can substantially improve efficacies in a broad range of applications. However, nanoparticle additives that are often used to do so can catastrophically destabilize a delicate emulsion system, in our case, a high internal phase emulsion (HIPE), whereas
Zhifeng Yi, Andrea Merenda, Lingxue Kong, Aleksandra Radenovic, Mainak Majumder, Ludovic F. Dumée,“Single step synthesis of Schottky-like hybrid graphene – titania interfaces for efficient photocatalysis”, : Scientific reports, 2018, 8, 8154
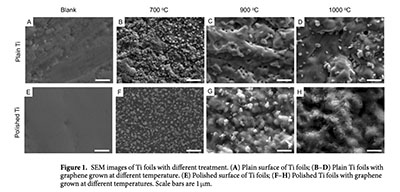
The development of 2D nanomaterial coatings across metal surfaces is a challenge due to the mismatch between the metal microstructure and the nanoscale materials. The naturally occurring thin oxidative layer present across all metal surfaces, may lead to low adherence and connectivity. In this paper, graphene/titania/Titanium hybrid flms were for the frst time fabricated by a single step chemical vapour deposition process across Titanium foils. The presence of graphene as a dopant was found to enhance the photocatalytic performance of the fnal products, applied to the degradation of organic molecules and to lead to Schottky-like junction formation at the metal/oxide interface. These Schottky junctions, where vacancies are present across the titania material due to the graphene doping and where Ti3+ ions are predominantly located, yield enhanced catalytic performance. The highest degradation rate was found to be 9.66×10−6min−1, achieved by the sample grown at 700°C for 5min, which was 62% higher than the sample just treated at that temperature without graphene growth. This work provides evidence that graphene may be grown across pure Titanium metal and opens new avenues in biomedical devices design, tribological or separation applications.
Abozar Akbari, Sally El Meragawi, Samuel T. Martin, Ben Corry, Ezzatollah Shamsaei, Christopher D. Easton, Dibakar Bhattacharyya, Mainak Majumder,“ Solvent Transport Behavior of Shear Aligned Graphene Oxide Membranes and Implications in Organic Solvent Nanofiltration
”, ACS Appl. Mater. Interfaces 2018, 10, 2067−2074
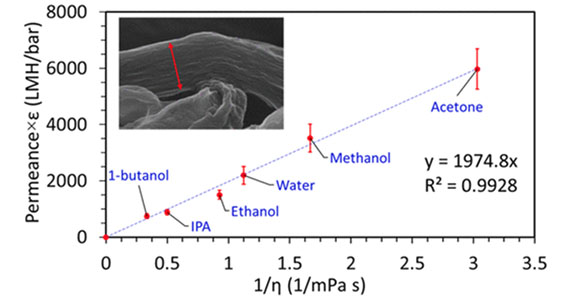
Solvent transport in membranes composed of stacked sheets of graphene oxide (GO) with molecular scale channels and a complex arrangement of hydrophobic and hydrophilic domains is not well understood. Here, we observe that the interlayer space between GO sheets expands in different solvents without disturbing the membrane integrity and is typically larger in aqueous media compared to nonaqueous media. However, the membranes have a tighter molecule sieving feature in aqueous media as demonstrated by lower permeance and higher solute rejection arising from interfacial water layers “sticking” to charged polar groups. As a result of this polar interaction, the permeance of polar solvents in GO membrane scales inversely to the polarity of the solvent, which is contrary to other polymeric and ceramic hydrophilic membranes and also scales inversely to the viscosity of solvents as per continuum expectations. We highlight the extended solvent-handling space of GO membranes, such as in polar protic, polar aprotic, and nonpolar solvents, demonstrating versatility over a commercial nanofiltration membrane, and we predict exciting new applications in advanced separation engineering.
M. Shaibani, S.J.D. Smith, P. Chakraborty Banerjee, K. Konstas, A. Zafari, D.E Lobo, M. Nazari, A. F Hollenkamp, M R Hill, M. Majumder “Framework-mediated synthesis of highly microporous onion-like carbon: energy enhancement in supercapacitors without compromising power”, J. Mater. Chem. A, 2017, Advance Article
High power technologies require the delivery of large amounts of energy in brief time periods – a demand that cannot be met by the current materials used in supercapacitors because they do not store charge efficiently at high discharge rates. To address this unmet challenge, high surface area graphitic carbons with a large fraction of micropores, for increased capacity, and high pore connectivity in addition to high conductivity, for fast current-switching response, are needed. Typically, the high temperatures required for the transformation of carbonaceous precursors into graphitic networks collapses the micropores, so that even the most promising morphology in a precursor is lost during carbonisation. Here we demonstrate that a high surface area diamondoid framework (PAF-1) under moderate temperatures (≤900 °C) undergoes topology-preserved conversion into a onion-like carbon (OLC) material which uniquely exhibits high specific surface area (1084 m2 g−1), narrow, predominantly 5 Å pores and high electrical conductivity (2.8 S cm−1) – we term this as microporous onion-like carbon (MOLC). We measure capacitance of 211 F g−1 at 5 mV s−1, and unprecedented 52% capacitance retention at ultrafast 2000 mV s−1 – this property is a direct consequence of the curved graphitic planes of the OLCs incorporating microporosity from the precursor structure. Despite the fact that the micropores in our OLC are small and narrow, we show that optimal matching of the electrolyte (on the basis of solvated ionic radii) to the pore size enables us to achieve high power and energy densities simultaneously (—e.g., 3.85 W h kg−1 can be extracted at 27.7 kW kg−1). Also, MOLC electrodes with high-mass loading (8 mg cm−2) demonstrate remarkable areal capacitances (≈3 F cm−2) over a varied range of high discharge currents, which is among the highest reported for supercapacitor electrodes.
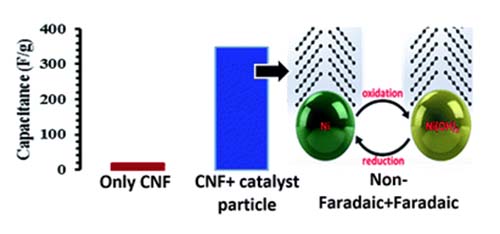
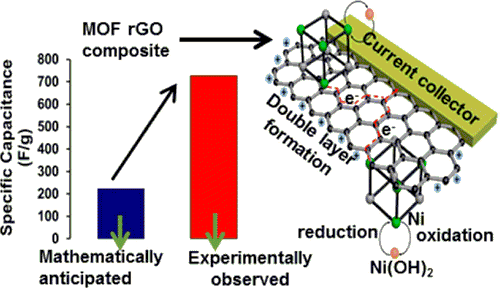
Parama Chakraborty Banerjee, Derrek E. Lobo, Tim Williams, Mahdokht Shaibani, Matthew R. Hill, Mainak Majumder, “Graphitic carbon nanofiber growth from catalytic-metal organic frameworks & their electrochemical double layer properties”, J. Mater. Chem. A, 2017,5, 25338-25349
Metal–organic frameworks have been widely studied as a template to nanoporous or well-structured carbon materials; however, utilizing the catalytic properties of MOFs to grow graphitic one dimensional nanostructures has been rarely reported. We have studied the evolution of carbon structures produced by transition metal doping (Ni in our case) in a Zn-based MOF and subjecting the MOF to a typical chemical vapour deposition process. Structures ranging from fine to thick graphitized carbon nanofibers are produced systematically at a relatively low temperature of 650 °C by increasing the Ni concentration in the MOF. A general trend of an increase in the nanofiber diameter with an increase in the Ni concentration and the formation of plate-like graphitic structures suggest that the catalyst particles undergo coalescence and impact the morphology of carbon structures produced. Without any further modification, the carbon nanofibers show modest electrical double layer (18 F g−1) behaviour in 1 M Na2SO4, but quite interestingly, the finely divided Ni particles undergo faradaic redox reactions in 1 M KOH and boost the capacitance to 348 F g−1. Our work suggests that our approach of utilizing catalytic MOFs for the growth of well-defined carbon nanostructures can serve more than one purpose towards the synthesis of hybrid materials for energy storage.
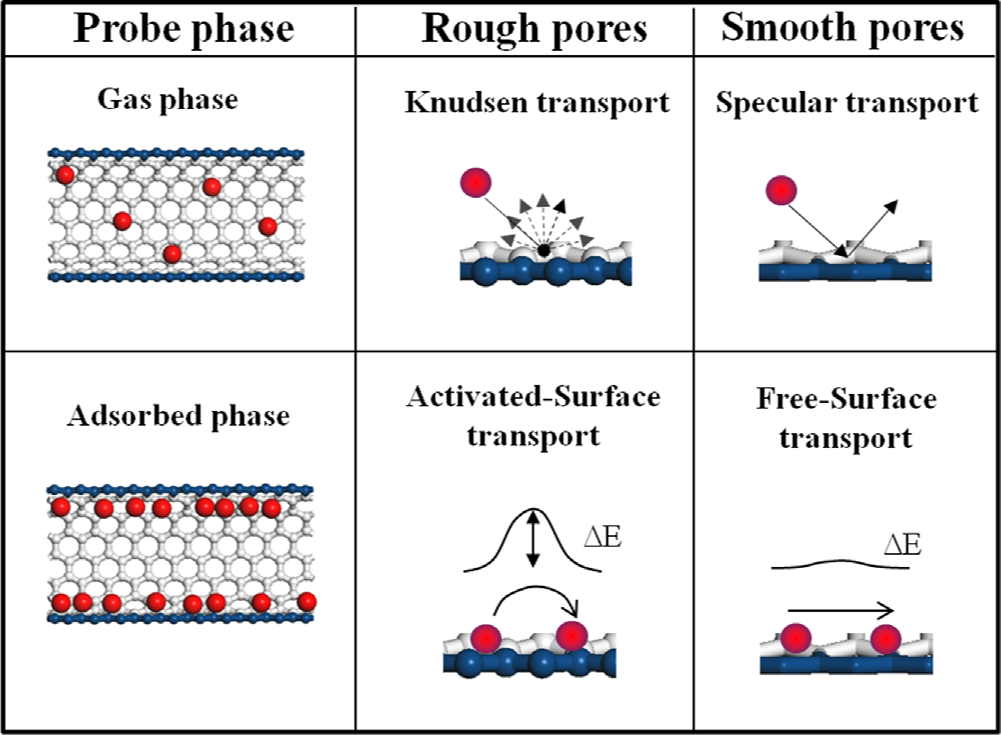
Mahdokht Shaibani, Anthony F Hollenkamp, Matthew R Hill, Mainak Majumder “Permselective membranes in lithium–sulfur batteries”, Current Opinion in Chemical Engineering 2017,16:31–38
Integration of permselective membranes – as a subset of the separator systems – in the configuration of lithium–sulfur battery, is a relatively simple solution to tackle the issue of polysulfide dissolution into the electrolyte. Cation-selective materials such as graphene oxide (GO) and Nafion have demonstrated to serve effectively as electrostatic shields for polysulfide anions, retarding their diffusion to the anode side of the battery. Looking into the future, the introducing of these materials in the configuration/composition of multifunctional/multilayered separator systems will promote the high efficiency cycling of high content sulfur cathodes. Enhanced performance metrics and careful minimisation of the mass/volume of these extra components should result in enhanced volumetric/gravimetric energy densities on a cell level for lithium–sulfur battery.
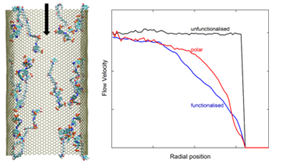
Mainak Majumder , Alessandro Siria , Lydéric Bocquet “Flows in one-dimensional and
two-dimensional carbon nanochannels: Fast and curious”, MRS Bulletin, 42, 2017, 278-282
Carbon materials exist in a large number of allotropic forms and exhibit a wide range of physical and chemical properties. From the perspective of fluidics, particularly within the confines of the nanoscale afforded by one-dimensional carbon nanotubes (CNTs) and two-dimensional graphene structures, many unique properties have been discovered. However, other questions, such as the link between electronic states and hydrodynamics and accurate model predictions of transport, remain unanswered. Theoretical studies, experiments in large-scale ensembles of CNTs and stacked graphene sheets, and precise measurements at the single-pore and single-molecule level have helped in our understanding. These activities have led to explosive growth in the field, now known as carbon nanofluidics. The ability to produce membranes and devices from fluid phases of graphene oxide, which retain these special properties in molecular-scale flow channels, promises realization of applications in the near term.
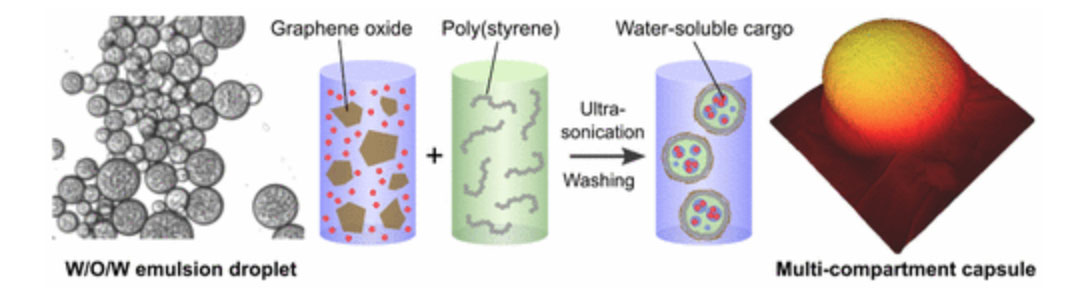
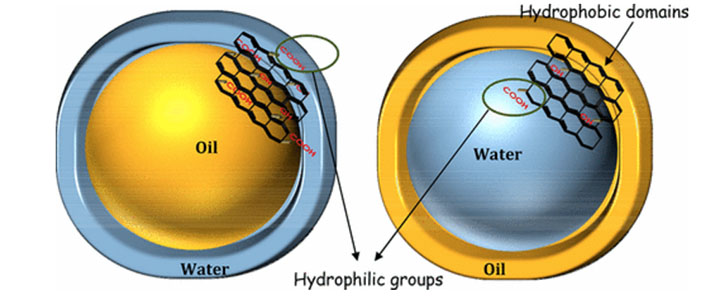
Muthana Ali, Thomas M. McCoy, Ian R. McKinnon, Mainak Majumder, Rico F. Tabor” Synthesis and Characterization of Graphene Oxide–Polystyrene Composite Capsules with Aqueous Cargo via a Water–Oil–Water Multiple Emulsion Templating Route”, ACS Appl. Mater. Interfaces, 2017, 9 (21), pp 18187–18198
Graphene oxide/polystyrene (GO/PS) nanocomposite capsules containing a two-compartment cargo have been successfully fabricated using a Pickering emulsion strategy. Highly purified GO sheets with typically micrometer-scale lateral dimensions and amphiphilic characteristics were prepared from the oxidation reaction of graphite with concomitant exfoliation of the graphite structure. These GO sheets were employed as a stabilizer for oil-in-water emulsions where the oil phase comprised toluene or olive oil. The stability and morphology of the emulsions were extensively studied as a function of different parameters including GO concentration, aqueous phase pH, ultrasonication time, effects of added electrolytes and stability to dilution. In selected conditions, the olive oil emulsions showed spontaneous formation of multiple w/o/w emulsions with high stability, whereas toluene formed simple o/w emulsions of lower overall stability. Olive oil emulsions were therefore used to prepare capsules templated from emulsion droplets by surrounding the oil phase with a GO/PS shell. The GO sheets, emulsions and composite capsules were characterized using a variety of physical and spectroscopic techniques in order to unravel the interactions responsible for capsule formation. The ability of the capsules to control the release of a model active agent in the form of a hydrophilic dye was explored, and release kinetics were monitored using UV–visible spectroscopy to obtain rate parameters. The composite capsules showed promising sustained release properties, with release rates 11× lower than the precursor GO-stabilized multiple emulsion droplets.
Tanesh D. Gamot, Arup Ranjan Bhattacharyya, Tam Sridhar, Fiona Beach, Rico F. Tabor, Mainak Majumder” Synthesis and Stability of Water-in-Oil Emulsion Using Partially Reduced Graphene Oxide as a Tailored Surfactant”, Langmuir, 2017, 33 (39),10311–10321
Graphene oxide (GO) is widely known as an amphiphile having hydrophilic oxygen functionality and unoxidized graphitic patches as the hydrophobic domains. Exploiting this amphiphilicity, GO serves as a surfactant to stabilize oil–water interfaces. While there are numerous reports on GO as a surfactant, most of these reports concern oil-in-water (O/W) emulsions, and there are very few on the formation of water-in-oil (W/O) emulsions. We prepared W/O emulsions using partially reduced graphene oxide (prGO) as a surfactant. The partial reduction introduces a subtle hydrophilic–lipophilic balance (HLB), which favors the formation of the W/O emulsion. The morphological features and rheological characteristics of the W/O emulsion with 75:25 water-to-oil ratio were investigated and analyzed in detail. The W/O emulsion was found to have polydispersity with wide range of droplet sizes varying between 2 to 500 μm. Using confocal microscopy, the role of parameters such as extent of reduction, continuous phase volume fraction and the concentration of GO on the stability, microstructure and variation of droplet size distribution of the W/O emulsion were carefully monitored. With prGO concentration as large as 0.05% (w/w), highly concentrated emulsion will form, and are stable up to 20 days from formation; destabilization occurred from sedimentation and subsequent coalescence as the partially reduced GO was limited by its dispersion ability in the oil-phase studied here. Understanding the mechanisms behind the transient stability will enable the development of novel emulsion compositions containing GO as a multifunctional additive.
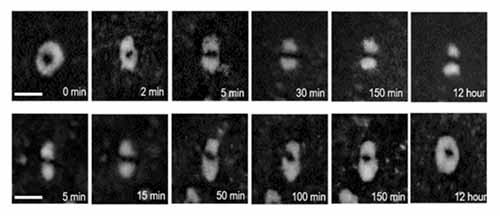
Rachel Tkacz, Md Joynul Abedin, Phillip Sheath, Shalin B. Mehta, Amitabh Verma, Rudolf Oldenbourg, and Mainak Majumder “Phase Transition and Liquid Crystalline Organization of Colloidal Graphene Oxide as a Function of pH”, Part. Part. Syst. Charact.2017, 34, 1600391
This paper discusses the influence of pH on colloidal graphene oxide (GO) liquid crystals. The results indicate that there is a crossing point where suspensions at pH > pKa, the GO sheets have different behavior than suspensions at pH < pKa. This behavior is evidenced by broadening of the biphasic region in the isotropic to nematic phase transition, by observing different shape and internal configurations of the nematic droplets, and in the magnitude of fractionation between large and small sheets in the nematic and isotropic phases. The behavior of GO liquid crystal droplets under the influence of a static magnetic field is studied, and evidence is found in the magnetically induced distortions for the support of our interpretation of the internal configurations of the nematic droplets. This study also demonstrates that magnetic distortions are reversible on a time scale of minutes to hours. This research is based on the LC‐PolScope, a polarized light system, which is found to be a powerful tool for characterization of GO liquid crystals due to its fast and easy image acquisition and analysis.
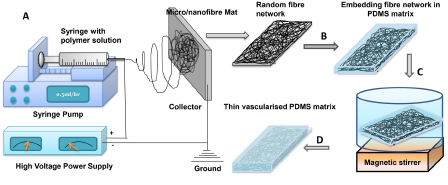
P. Kumar, P.S Gandhi and M. Majumder, “Interfacing 3D micro/nanochannels with a branch-shaped reservoir enhances fluid and mass transport”, J. Micromech. Microeng. 27 (2017) 015026 (11pp)
Three-dimensional (3D) micro/nanofluidic devices can accelerate progress in numerous fields such as tissue engineering, drug delivery, self-healing and cooling devices. However, efficient connections between networks of micro/nanochannels and external fluidic ports are key to successful applications of 3D micro/nanofluidic devices. Therefore, in this work, the extent of the role of reservoir geometry in interfacing with vascular (micro/nanochannel) networks, and in the enabling of connections with external fluidic ports while maintaining the compactness of devices, has been experimentally and theoretically investigated. A statistical modelling suggested that a branch-shaped reservoir demonstrates enhanced interfacing with vascular networks when compared to other regular geometries of reservoirs. Time-lapse dye flow experiments by capillary action through fabricated 3D micro/nanofluidic devices confirmed the connectivity of branch-shaped reservoirs with micro/nanochannel networks in fluidic devices. This demonstrated a ~2.2-fold enhancement of the volumetric flow rate in micro/ nanofluidic networks when interfaced to branch-shaped reservoirs over rectangular reservoirs. The enhancement is due to a ~2.8-fold increase in the perimeter of the reservoirs. In addition, the mass transfer experiments exhibited a ~1.7-fold enhancement in solute flux across 3D micro/nanofluidic devices that interfaced with branch-shaped reservoirs when compared to rectangular reservoirs. The fabrication of 3D micro/nanofluidic devices and their efficient interfacing through branch-shaped reservoirs to an external fluidic port can potentially enable their use in complex applications, in which enhanced surface-to-volume interactions are desirable.
S.T. Martin, A. Akbari, P. Chakraborty Banerjee, A. Neild and M.Majumder “The inside-out supercapacitor: induced charge storage in reduced graphene oxide “, Phys. Chem. Chem. Phys., 2016,18, 32185-32191
Iontronic circuits are built using components which are analogous to those used in electronic circuits, however they involve the movement of ions in an electrolyte rather than electrons in a metal or semiconductor. Developments in these circuits’ performance have led to applications in biological sensing, interfacing and drug delivery. While transistors, diodes and elementary logic circuits have been demonstrated for ionic circuits if more complex circuits are to be realized, the precident set by electrical circuits suggests that a component which is analogous to an electrical capacitor is required. Herein, an ionic supercapacitor is reported, our experiments show that charge may be stored in a conductive porous reduced graphene oxide film that is contacted by two isolated aqueous solutions and that this concept extends to an arbitrary polarizable sample. Parametric studies indicate that the conductivity and porosity of this film play important roles in the resultant device’s performance. This ionic capacitor has a specific capacitance of 8.6 F cm−3 at 1 mV s−1 and demonstrates the ability to filter and smooth signals in an electrolyte at a variety of low frequencies. The device has the same interfaces as a supercapacitor but their arrangement is changed, hence the name inside-out supercapacitor
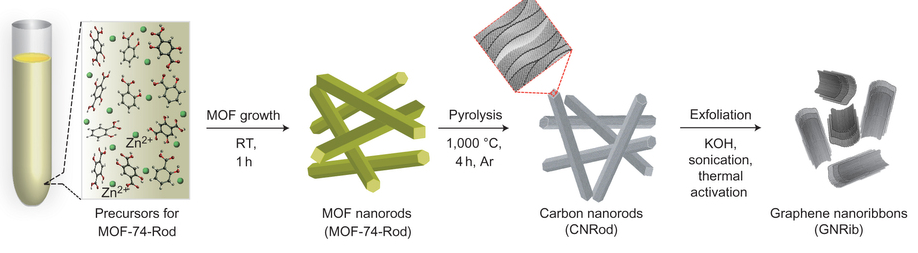
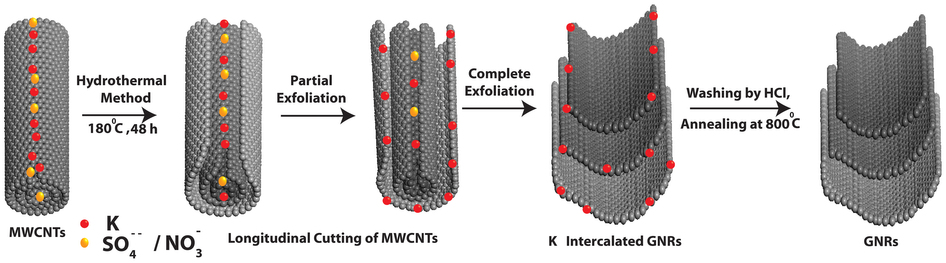
SP. Pachfule, D.Shinde, M. Majumder & Q.Xu “Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework, Nature Chemistry 8, 718–724 (2016) ,
One- and two-dimensional carbon nanomaterials are attracting considerable attention because of their extraordinary electrical, mechanical and thermal properties, which could lead to a range of important potential applications. Synthetic processes associated with making these materials can be quite complex and also consume large amounts of energy, so a major challenge is to develop simple and efficient methods to produce them. Here, we present a self-templated, catalyst-free strategy for the synthesis of one-dimensional carbon nanorods by morphology-preserved thermal transformation of rod-shaped metal–organic frameworks. The as-synthesized non-hollow (solid) carbon nanorods can be transformed into two- to six-layered graphene nanoribbons through sonochemical treatment followed by chemical activation. The performance of these metal–organic framework-derived carbon nanorods and graphene nanoribbons in supercapacitor electrodes demonstrates that this synthetic approach can produce functionally useful materials. Moreover, this approach is readily scalable and could be used to produce carbon nanorods and graphene nanoribbons on industrial levels.
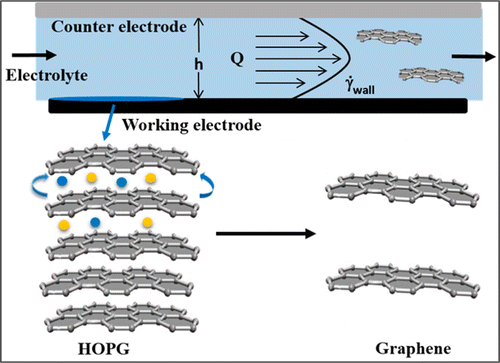
D.B. Shinde, J. Brenker, C. D. Easton, R. F. Tabo , A. Neild, and M. Majumder,”Shear Assisted Electrochemical Exfoliation of Graphite to Graphene”, Langmuir, 2016, 32 (14), pp 3552–3559
The exfoliation characteristics of graphite as a function of applied anodic potential (1–10 V) in combination with shear field (400–74 400 s–1) have been studied in a custom-designed microfluidic reactor. Systematic investigation by atomic force microscopy (AFM) indicates that at higher potentials thicker and more fragmented graphene sheets are obtained, while at potentials as low as 1 V, pronounced exfoliation is triggered by the influence of shear. The shear-assisted electrochemical exfoliation process yields large (∼10 μm) graphene flakes with a high proportion of single, bilayer, and trilayer graphene and small ID/IG ratio (0.21–0.32) with only a small contribution from carbon–oxygen species as demonstrated by X-ray photoelectron spectroscopy measurements. This method comprises intercalation of sulfate ions followed by exfoliation using shear induced by a flowing electrolyte. Our findings on the crucial role of hydrodynamics in accentuating the exfoliation efficiency suggest a safer, greener, and more automated method for production of high quality graphene from graphite..
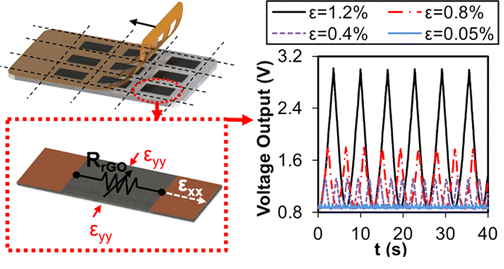
M. B. Coskun, A. Akbari, D.T. H. Lai, A. Neild, M. Majumder, T. Alan “Ultrasensitive Strain Sensor Produced by Direct Patterning of Liquid Crystals of Graphene Oxide on a Flexible Substrate”, ACSAppl.Mater.Interfaces 2016, 8, 22501 − 22505
Ultrasensitive flexible strain sensors were developed through the combination of shear alignment of a high concentration graphene oxide (GO) dispersion with fast and precise patterning of multiple rectangular features on a flexible substrate. Resistive changes in the reduced GO films were investigated under various uniaxial strain cycles ranging from 0.025 to 2%, controlled with a motorized nanopositioning stage. The devices uniquely combine a very small detection limit (0.025%) and a high gauge factor with a rapid fabrication process conducive to batch production.
M.Shaibani, A.Akbari, P.Sheath, C.D. Easton, P.Chakraborty-Banerjee, K.Konstas, A. Fakhfouri, M. Barghamadi, M. M. Musameh, A.S. Best, T.Ruther, P.J. Mahon, M.R.Hill, A.F. Hollenkamp, M.Majumder, “Supressed Polysulfide Crossover in Li-S Batteries through a High-Flux Graphene Oxide Membrane Supported on a Sulfur Cathode”, ACS Nano, 2016, 10 (8), pp 7768–7779
Utilization of permselective membranes holds tremendous promise for retention of the electrode-active material in electrochemical devices that suffer from electrode instability issues. In a rechargeable Li–S battery—a strong contender to outperform the Li-ion technology—migration of lithium polysulfides from the sulfur cathode has been linked to rapid capacity fading and lower Coulombic efficiency. However, the current approaches for configuring Li–S cells with permselective membranes suffer from large ohmic polarization, resulting in low capacity and poor rate capability. To overcome these issues, we report the facile fabrication of a high-flux graphene oxide membrane directly onto the sulfur cathode by shear alignment of discotic nematic liquid crystals of graphene oxide (GO). In conjunction with a carbon-coated separator, the highly ordered structure of the thin (∼0.75 μm) membrane and its inherent surface charge retain a majority of the polysulfides, enabling the cells to deliver very high initial discharge capacities of 1063 and 1182 mAh gelectrode–1 for electrodes with 70 and 80% sulfur content, respectively, at the practical 0.5 C rate. The very high sulfur utilization and impressive capacity retentions of the high sulfur content electrodes result in some of the highest performance metrics in the literature of Li–S (e.g., electrode capacity of 835 mAh gelectrode–1 after 100 cycles at 0.5 C with a sulfur content of 80%). We show that the structural order of the shear-aligned GO membrane is key in maintaining good kinetics of the charge transfer processes in Li–S batteries.
A.Akbari, P.Sheath, D.B. Shinde, M.Shaibani, P.C. Banerjee, R.Tkacz, D.Bhattacharyya, M.Majumder, “Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide”, Nature Communications, 7, Article number 10891, doi:10.1038/ncomms10891
Graphene-based membranes demonstrating ultrafast water transport, precise molecular sieving of gas and solvated molecules shows great promise as novel separation platforms; however, scale-up of these membranes to large-areas remains an unresolved problem. Here we demonstrate that the discotic nematic phase of graphene oxide (GO) can be shear aligned to form highly ordered, continuous, thin films of multi-layered GO on a support membrane by an industrially adaptable method to produce large-area membranes (13 × 14 cm2) in <5 s. Pressure driven transport data demonstrate high retention (>90%) for charged and uncharged organic probe molecules with a hydrated radius above 5 Å as well as modest (30–40%) retention of monovalent and divalent salts. The highly ordered graphene sheets in the plane of the membrane make organized channels and enhance the permeability (71±5 l m−2 hr−1 bar−1 for 150±15 nm thick membranes).
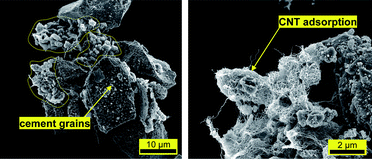
S.J.Chen, W.Wang, K.S. Crentsii, F.Collins, X.L. Zhao, M.Majumder, W.H. Duan, “Distribution of carbon nanotubes in fresh ordinary Portland cement pastes: understanding from a two-phase perspective”, RSC Adv., 2016,6, 5745-5753,
Significant research advances have been made in the field of carbon nanotube (CNT) reinforced ordinary Portland cement (OPC) paste composites in recent years. However, the distribution of CNTs in fresh OPC paste is yet to be fully researched and quantified, thereby creating a technical barrier to CNT utilization in concrete construction. In this study, fresh OPC paste was treated as a two-phase material containing solid particles (cement grains) and liquid solutions (pore solutions). A centrifugation-based technique was proposed to separate these two phases and the presence of CNTs in each phase was quantified. UV-Vis spectrometry showed that the degree of dispersion can achieve above 90 wt% using polycarboxylate superplasticizer. The results suggested an upper limit of 0.26 wt% for CNT addition into water before mixing with OPC, and the dispersion was found to be stable for at least 4 hours. Based on scanning electron imaging, the adsorption phenomenon of CNTs on OPC grains with size less than 4 μm was discovered. Energy-dispersive X-ray spectroscopy indicated these adsorptive particles have lower Ca to Si ratio. It was observed that about 0.5 mg of CNTs per gram of OPC grains was adsorbed in solid OPC grains in typical fresh OPC pastes. On the basis of these results, a conceptual model was proposed for the distribution of CNTs in fresh OPC paste where about 33 wt% of the CNTs stay in pore solution and 65 wt% of CNTs are adsorbed on OPC grains.
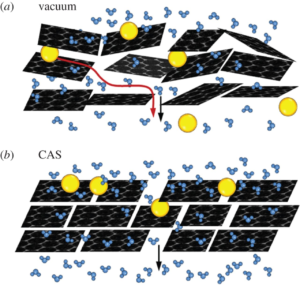
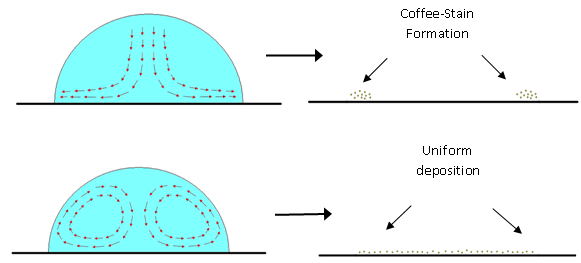
P.Sheath & M.Majumder, “Flux accentuation and improved rejection in graphene-based filtration membranes produced by capillary-force-assisted self-assembly, “, Phil.Trans.R.Soc.A, 2015, 374: 20150028
Separation and flux performance were compared in graphene-based membranes that differed only in the method of deposition of reduced graphene oxide platelets. Membranes with higher degree of order were produced by evaporation-induced capillary-force self-assembly, which showed higher steric rejection properties while simultaneously accentuating water permeance compared to membranes produced by the traditional vacuum filtration technique. These studies attempt to establish structure–property correlations in graphene-based membranes.
D.E. Lobo, P.C. Banerjee-Chakraborty, CD. Easton, M.Majumder, ” Miniaturized Supercapacitors: Focused Ion Beam Reduced Graphene Oxide with Enhanced performance Metrics”, Adv. Energy Mater. 2015, 5, 1500665
Miniaturization of energy storage devices with enhanced performance metrics can reduce the footprint of microdevices being used in our daily life. Micro-supercapacitor architectures with planar geometry provides several advantages, such as, the ability to control and reduce the distances ions travel between two electrodes, easy integration to microdevices, and offer the potential of being extended into 3D without compromising the interelectrode distances. Here, focused ion beam (FIB) technology is used to directly write miniaturized planar electrode systems of reduced graphene oxide (FIB-rGO) on films of graphene oxide. Using optimized ion beam irradiation, interdigitated FIB-rGO electrode designs with 40 μm long and 3.5 μm wide fingers with ultrasmall interelectrode spacing of 1 μm demonstrate a large capacitance (102 mF cm−2), ultrasmall time response (0.03 ms), low equivalent series resistance (0.35 mΩ cm2), and retain 95% of the capacitance after 1000 cycles at an ultrahigh current density of 45 mA cm−2. These performance metrics show remarkable improvements on several counts of supercapacitor performance over existing reports due to the miniaturized electrode dimensions and minimal damage to the graphene sheets. It is believed that these results can provide avenues for large-scale fabrication of arrayed, planar, high-performance micro-supercapacitors with a small environmental footprint.
Above: Cover Image in Advanced Energy Materials
A.W. Thornton, A.Ahmed, S.K. Kannam, B.D. Todd, M.Majumder, A.J. Hill, ” Analytical Diffusion Mechanism (ADiM) model combining specular, Knudsen and surface diffusion”, J.Memb.Sci., 485, 1-9, 2015
We present a unified transport model that combines specular, Knudsen and surface diffusion mechanisms, termed the Analytical Diffusion Mechanism (ADiM) model. The ADiM model uniquely describes the transport behaviour of the bulk gas and adsorbed phase taking place in rough and smooth nanopores. Experiments and molecular simulations of nitrogen flow through aligned nanotube-based membranes are used to verify the model. In addition, we explore entrance effects using a suction energy mechanism that is compatible with ADiM and can accelerate gas permeance by an order of magnitude. Finally, ADiM is used to assess the effect of tube size on post-combustion carbon dioxide separation from fossil fuel plants.
L.Dumee, L.He, Z.Wang, P.Sheath, J. Xiong, C.Feng, M.Y. Tan, F.She, M.Duke, S. Gray, A.Pacheco, P.Hodgson, M.Majumder, L. Kong, “Growth of nano-textured graphene coatings across highly porous stainless steel supports towards corrosion resistant coatings” Carbon, 87, 395-408, 2015
In this paper, we demonstrated for the first time the growth of 3D networks of graphene nano-flakes across porous stainless steel substrates of micron sized metal fibres, and its anti-corrosion properties. The controlled formation of graphene-grade coatings in the form of single sheets to complex and homogeneously distributed 2–4 μm long nano-pillars is demonstrated by Scanning Electron Microscopy. The morphology and stability of these structures are shown to be particularly related to the temperature and feed gas flow rate during the growth. The number of layers across the graphene materials was calculated from the Raman spectra and is shown to range between 3 and more than 15 depending on the growth conditions and to be particularly related to the time and flow rate of the experiment. The presence of the graphene was shown to massively enhance the specific surface area of the material and to contribute to the increased corrosion resistance and electrical conductivity of the material without compromising the properties or structure of the native stainless steel materials. This new approach opens up a new route to the facile fabrication of advanced surface coatings with potential applications in developing new thermal exchangers, separation and bio-compatible materials.
R. Khare, D.B. Shinde, S.Bansode, M.A. More, M.Majumder, V.K. Pillai, D.J. Late, “Graphene nanoribbons as prospective field emitter” Appl. Phys. Lett. 106, 023111, 2015
Field emission characteristics of graphene nanoribbons (GNRs) synthesized by unzipping of multiwall carbon nanotubes using a facile hydrothermal route have been investigated at a base pressure of 1 × 10−8 mbar. The values of turn-on field, required to draw an emission current densities of 1 and 10 μA/cm2, are found to be 2.8 and 5.8 V/μm, respectively, and a maximum emission current density of 500 μA/cm2has been drawn at an applied field of 9.8 V/μm. The emission current stability of the GNRs emitter was studied at preset values of 1 and 10 μA over a period of 3 h, and is found to be excellent. The field emission results demonstrated herein suggest that GNRs based field emitters can open up many opportunities for their potential utilization as large area field emitters in various vacuum micro-nanoelectronic devices such as flexible field emission displays, portable X-ray, and microwave tubes.
P.Chakraborty-Banerjee, Derrek Lobo, R. Middag, W.K. Ng, Mahdokht E. Shaibani and Mainak Majumder , “Electrochemical Capacitance of Ni-doped Metal Organic Framework and Reduced Graphene Oxide Composites: More than the Sum of its Parts” ACS Appl. Mater. Interfaces, 2015, 7 (6), pp 3655–3664
Composites of a Ni-doped metal organic framework (MOF) with reduced graphene oxide (rGO) are synthesized in bulk (gram scale) quantities. The composites are composed of rGO sheets, which avoid restacking from the physical presence of MOF crystals. At larger concentration of rGO, the MOF crystals are distributed on the overlapping and continuous rGO sheets. Ni in Ni-doped MOF is found to engage in a two-electron, reversible, efficient, redox reaction shuttling between Ni and Ni(OH)2 in aqueous potassium hydroxide (KOH) electrolyte. The reaction is rather unique as Ni-based supercapacitors use a one-electron transfer Faradaic redox reaction between Ni(OH)2 and NiO(OH). Employing electrochemical impedance spectroscopy, we determined the charge transfer resistance to be 184 mΩ for MOF, 74 mΩ for a Ni-doped MOF and 6 mΩ for a rGO–Ni-doped MOF composite, but these modifications do not affect the mass transfer resistance. This novel redox reaction in conjunction with the lowered charge transfer resistance from the introduction of rGO underpins the synergy that dramatically increases the capacitance to 758 F/g in the rGO–Ni-doped MOF composite, when the parent MOF could store only 100 F/g and a physical composite of rGO and Ni-doped MOF could algebraically achieve about 240 F/g. A generic approach of doping MOFs with a redox active metal and forming a composite with rGO transforms an electro-inactive MOF to high capacity energy storage material with energy density of 37.8 Wh/kg at a power density of 227 W/kg. These results can promote the development of high-performance energy storage materials from the wide family of MOFs available.
M.Miansari, J.R.Friend. P.Chakraborty-Banerjee, M.Majumder, L.Y.Yeo, “Graphene-based planar nanofluidic rectifier”, J. Phys. Chem. C, 2014, 118 (38), pp 21856–21865
Structurally symmetric two-dimensional multilayered graphene oxide films, which facilitate ion transport through ‘nanochannels’ comprising the interstitial spaces between each stacked sheet within the film, are for the first time shown to exhibit peculiar ion current rectification and nonlinear current-voltage characteristics below a critical electrolyte concentration when the interstitial spacing becomes comparable to the Debye screening length such that the film becomes permselective. We attribute the unexpected rectification behavior to the fore-aft asymmetry that arises in the diffusion boundary layer on both sides of the millimeter long film upon reversal between the high resistance positive bias state to the low resistance negative bias state; the asymmetry being primarily a consequence of the trapping and release of counter-ions within the film, compounded by the nonuniform electric field that occurs in the tortuous nanochannels within the film. In addition to elucidating the influence of the electrolyte concentration and applied bias voltage, we demonstrate the possibility of tuning the ion selectivity and hence the rectification behavior through the solution pH. These first graphene-based nanofluidic rectifiers, which are easily synthesized, therefore offer a flexible, robust, low cost and facile large-scale alternative to conventional nanochannels that require elaborate and sophisticated nanofabrication.
S.Martin, A.Neild, M.Majumder,”Graphene-based ion rectifier using macroscale geometric asymmetry”, APL Mat. 2, 092803, (2014) – Special Topic in 2D Materials
Ion rectification is the asymmetrical conduction of ions through a system under different polarities of applied potentials. In this article we report the finding of a novel form of ion rectification in graphene oxide (GO) and reduced graphene oxide (RGO) films which act as an ensemble array of nanochannels. Rectification is imparted by introducing geometric asymmetry in fluidic inlets to the counter-ion selective nanochannels of GO/RGO which creates asymmetry in the enrichment/depletion effects at the macro-/nano-interface. The devices are made simply by cutting a GO or RGO film into a trapezoid and sealing the film within a Polydimethylsiloxane (PDMS) block so that fluid may only enter through one of two inlets. These devices exhibit rectification ratios larger than 20 (in 1 mM NaCl) while operating at modest voltages [-1V,+1V].
S.J. Chen, B.Zou, F.Collins, X.L. Zhao, M.Majumder, W.H.Duan,”Predicting the influence of ultrasonication energy on the reinforcing efficiency of carbon nanotubes”, Carbon, 2014
Ultrasonication is extensively used in the fabrication of carbon nanotube (CNT) reinforced composites. However, there has been very limited research into predicting the effect of ultrasonication treatment on the reinforcing efficiency of CNTs. In this study, a theoretical framework with supporting experiments is developed to address this issue. The distribution of CNT lengths and the concentration of dispersed CNTs are characterized using scanning electron microscopy images and UV-vis spectra. After ultrasonication, the length of CNT is found to follow log-normal distributions which show a shortening effect. The concentration of dispersed CNT increases with ultrasonication energy but reaches a plateau after about 250 J/ml. The distribution of CNT lengths and the concentration of dispersed CNTs are incorporated into a micromechanics-based model to simulate the crack bridging behavior of CNTs. Results show that the distribution of CNT lengths leads to better estimation of reinforcing effect than does the average length. Furthermore, for unit volume of dispersed CNTs, the reinforcing efficiency decreases monotonically with increased ultrasonication. Based on the proposed model, the predicted optimal ultrasonication energy (89 J/ml) for reinforcing is found before the dispersion plateau is reached.
R.Tkacz, R.Oldenbourg, S.B. Mehta, A. Verma M.Miansari, M.Majumder,”pH Dependent Isotropic to Nematic Phase Transitions in Graphene Oxide Dispersions Reveal Droplet Liquid Crystalline Phases”, Chem. Commun., 2014,50, 6668-6671
Size fractionation, amplified by the surface charge density of graphene oxide (GO) sheets, broadens the pH dependent isotropic (I) to nematic (N) phase transition in aqueous dispersions of graphene oxide (GO). In this biphasic region, a highly organized droplet nematic phase of uniform size (20 ± 2.8 μm diameter) with an isotropic interior is observed.
D.B. Shinde, M.Majumder, V.K. Pillai,”Counter-Ion Dependent Longitudinal Unzipping of Multi-Walled Carbon Nanotubes to Highly Conductive and Transparent Carbon Nanoribbons”, Scientific Reports 4, Article # 4363
Here we report for the first time, a simple hydrothermal approach for the bulk production of highly conductive and transparent graphene nanoribbons (GNRs) using several counter ions from K2SO4, KNO3, KOH and H2SO4 in aqueous media, where, selective intercalation followed by exfoliation gives highly conducting GNRs with over 80% yield. In these experiments, sulfate and nitrate ions act as a co-intercalant along with potassium ions resulting into exfoliation of multi-walled carbon nanotubes (MWCNTs) in an effective manner. The striking similarity of experimental results in KOH and H2SO4 that demonstrates partially damaged MWCNTs, implies that no individual K+, SO42− ion plays a key role in unwrapping of MWCNTs, rather this process is largely effective in the presence of both cations and anions working in a cooperative manner. The GNRs can be used for preparing conductive 16 kΩsq−1, transparent (82%) and flexible thin films using low cost fabrication method.
R.Tkacz, R.Oldenbourg, A.Fulcher, M.Miansari, M.Majumder,”Capillary-Force Assisted Self-Assembly (CAS) of highly Ordered and Anisotropic Graphene-Based Thin Films”, J.Phys.Chem.C, 2014, 118 (1), 259–267
We report capillary-force-assisted self-assembly (CAS) as a method for preparation of thin films of chemically reduced graphene oxide (rGO) with unidirectional organization of rGO sheets. The films were initiated at the contact line of the air–liquid–solid interface and form directly on solid substrates dipped in an isotropic colloidal suspension of rGO. Assisted by capillary forces at the contact line, the suspension undergoes an isotropic-to-anisotropic phase transition and becomes aligned with the film growth direction as the contact line moves across the substrate surface. We determined the degree of order in rGO films and assemblies by birefringence and diattenuation imaging. The slow axis of the rGO platelets within the CAS films displayed a narrow angular distribution (±3°) within a film area of 1 mm2, resulting in the highest possible order parameter (S) of 1 with 8-fold enhancement of electrical conductivity compared to films formed by traditional techniques such as filtration. Our straightforward film fabrication technique is scalable to produce large areas of films, and by controlling the rates of convective to diffusive mass transport, films with varying degree of order can be produced.
A.W.K. Ma, J.Nam, N.Behabtu, F.Mirri, C.C. Young, B.Dan, D. Tsentalovich, M.Majumder, L.Song, Y.Cohen, P.M. Ajayan, M.Pasquali, “Scalable Formation of Carbon Nanotube Films Containing Highly Aligned Whiskerlike Crystallites”, Ind. Eng. Chem. Res., 2013, 52 (26), pp 8705–8713
We report the creation of carbon nanotube films from superacids by a scalable process, where film morphology is controlled by initial fluid phases. These films were formed by dip-coating biphasic (isotropic + liquid crystalline) carbon nanotube (CNT) chlorosulfonic acid solutions. Chlorosulfonic acid has low volatility and is therefore removed by solvent extraction instead of conventional drying processes. At intermediate concentrations, the solutions contain liquid crystalline domains which stretch and align streamwise during dip-coating. These elongated domains further act as “nucleating sites” for large, aligned whiskerlike crystallites during subsequent solvent extraction. The final films contain highly aligned CNT crystallites embedded in a mesh of randomly oriented CNTs.
M.Majumder, P.Sheath, J.I. Mardel, T.G. Harvey, A.W. Thornton, A.Gonzago, D.F. Kennedy, I.Madsen, J.W. Taylor, D.R. Turner, M.R. Hill “Aqueous Molecular Sieving and Strong Gas Adsorption in Highly Porous MOFs with a Facile Synthesis”, Chem. Mater. 2012, 24 (24), pp 4647–4652
Aqueous molecular sieving is demonstrated in a new series of isostructural metal organic frameworks based on the perylene tetracarboxylate (PTC) ligand. The frameworks can be formed in water at room temperature with Mg, Ni, and other first row transition metal ions and adopt a highly porous topology that results in predicted surface areas of over 2000 m2 g–1 and periodic channels of around 6 Å in diameter. Unusually, the M-PTC MOFs are highly resistant to moisture and can be readily synthesized on multigram scales. The frameworks have been shown to exhibit molecular sieving in the absorption from mixtures of organic molecules at low aqueous concentrations, with an application demonstrated on a dangerous water-borne herbicide, Paraquat. Ni-PTC also exhibits a structural flexibility that leads to strong and selective gas adsorption characteristics, with an IAST selectivity of 300 for carbon dioxide being adsorbed over nitrogen. Binding enthalpies for hydrogen and carbon dioxide are also very strong in comparison to other MOFs, at 10.75 and 52.50 kJ/mol respectively.
D. Lobo, J.Fu, T. Gengenbach, and M. Majumder “Localized Deoxygenation and Direct Patterning of Graphene Oxide Films by Focussed Ion Beams”, Langmuir, 2012, 28, 41,14815–14821
Exposure to controlled doses(~4.65×10-3 to 2.79×10-2 nC/µm2 ion fluence) of Ga ions via a focused ion beam (FIB) deoxygenates graphene oxide (GO) and increases the electrical conductivity in 100 × 100 μm patches by several orders of magnitudes compared to the unexposed GO. Raman spectra and carbon/oxygen ratio in exposed areas are indicative of chemically reduced graphene oxide (rGO). This novel FIB-induced conversion technique is harnessed for direct imprinting of complex micron-scale shapes and sub-20 nm lines of rGO in insulating films and flakes of GO establishing the capability for generating features varying in sizes from ~10’s nm to ~100’s of μm, in a mask-less and efficient manner.
M. Majumder, Clint s Rendall, J. Alexander Eukel, James Y.L Wang, Natnael Behabtu, Cary Pint, Tzu-Yu Liu, Alvin W Orbaek, Francesca Mirri, Jaewook Nam, Andrew R Barron, Robert H. Hauge, Howard K. Schmidt, and Matteo Pasquali “Overcoming the ‘Coffee-Stain Effect by Compositional Marangoni Flow Assisted Drop-Drying”, J.Phys.Chem.B, 2012, 116 (22), pp 6536–6542
Attempts at depositing uniform films of nanoparticles by drop-drying have been frustrated by the “coffee-stain” effect, due to convective macroscopic flow into the contact line. Here we show that uniform deposition of nanoparticles in aqueous suspensions can be attained easily by drying the droplet in an ethanol vapor atmosphere. This technique allows the particle-laden water droplets to spread on a variety of surfaces such as glass, silicon, mica, PDMS, and even Teflon®. Visualization of droplet shape and internal flow shows initial droplet spreading and strong re-circulating flow during spreading and shrinkage. The initial spreading is due to diminishing contact angle from the absorption of ethanol from the vapor at the contact line. During the drying phase, the vapor is saturated in ethanol, leading to preferential evaporation of water at the contact line. This generates a surface tension gradient which drives a strong recirculating flow and homogenizes the nanoparticle concentration. We show that this method can be used for depositing catalyst nanoparticles for the growth of single-walled carbon nanotubes as well as to manufacture plasmonic films of well-spaced, unaggregated gold nanoparticles.
R.K. Singh Raman, P. Chakraborty Banerjee, Derrek E. Lobo, Hemtej Gullapalli, Madusha Sumandasa, Ashwin Kumar, Lokesh Choudhary, Rachel Tkacz, Pulickel M. Ajayan, M.Majumder “Protecting Copper from Electrochemical Degradation by Graphene Coating ” Carbon, 2012, 50,11,4040–4045
Graphene coating on copper (Cu) is shown to increase the resistance of the metal to electrochemical degradation by one and half orders of magnitude. Detailed electrochemical characterization in aggressive chloride environment shows the impedance of Cu increasing dramatically and the anodic and cathodic current densities of the coated Cu becoming nearly 1–2 orders of magnitude smaller when coated with graphene. The observations are counterintuitive as graphite in contact with metals increases metallic corrosion. The results can bring paradigm changes in the development of anti-corrosion coatings using conformal, ultra thin graphene films.
Ji Wu, Karen Gerstandt, M.Majumder, Xin Zhan and Bruce J. Hinds “Highly efficient electroosmotic flow through functionalized carbon nanotube membranes ” Nanoscale, 2011, 3, 3321-3328
Carbon nanotube membranes with inner diameter ranging from 1.5–7 nm were examined for enhanced electroosmotic flow. After functionalization via electrochemical diazonium grafting and carbodiimide coupling reaction, it was found that neutral caffeine molecules can be efficiently pumped via electroosmosis. An electroosmotic velocity as high as 0.16 cm s−1 V−1 has been observed. Power efficiencies were 25–110 fold improved compared to related nanoporous materials, which has important applications in chemical separations and compact medical devices. Nearly ideal electroosmotic flow was seen in the case where the mobile cation diameter nearly matched the inner diameter of the single-walled carbon nanotube resulting in a condition of using one ion is to pump one neutral molecule at equivalent concentrations.
W.Gao, M. Majumder, L. Alemany, T. Narayanan, M. Ibarra, B.K. Pradhan, P.M. Ajayan “Engineered Graphite Oxide Materials for Application in Water Purification” ACS Appl. Mater. Interfaces, 2011, 3 (6), pp 1821-1826
Retaining the inherent hydrophilic character of GO (graphite-oxide) nanosheets, sp2 domains on GO are covalently modified with thiol groups by diazonium chemistry. The surface modified GO adsorbs 6-fold higher concentration of aqueous mercuric ions than the unmodified GO. “Core-shell” adsorbent granules, readily useable in filtration columns, are synthesized by assembling aqueous GO over sand granules. The nanostructured GO-coated sand retains at least 5-fold higher concentration of heavy metal and organic dye than pure sand. The research results could open avenues for developing low-cost water purification materials for the developing economies.
M.Majumder and B.Corry, “Anomalous Decline of Water Transport in Covalently Modified Carbon Nanotube Membranes”, Chem. Commun., 2011, 47, 7683-7685
Carbon nanotube membranes have been shown to rapidly transport liquids; but progressive hydrophilic modification-contrary to expectations-induces a drastic reduction of water flow. Enhanced electrostatic interaction and the disruption of the mechanically smooth graphitic walls is the determinant of this behavior. These results have critical implications in the design of nanofluidic devices.
M.Majumder and P.M. Ajayan “Carbon Nanotube Membranes: A new frontier in membrane science” in: Enrico Drioli and Lidietta Giorno Comprehensive Membrane Science and Engineering (Elsevier Science)
M. Majumder, N.Chopra, B.J. Hinds, “Mass Transport through Carbon Nanotube Membranes in Three Different Regimes: Ionic Diffusion and Gas and Liquid Flow” ACS Nano, 2011, 5, 3867-78
Transport phenomena through the hollow conduits of carbon nanotubes (CNTs) are subjects of intense theoretical and experimental research. We have studied molecular transport over the large spectrum of ionic diffusion to pressure-driven gaseous and liquid flow. Plasma oxidation during the fabrication of the membrane introduces carboxylic acid groups at the CNT entrance, which provides electrostatic “gatekeeper” effects on ionic transport. Diffusive transport of ions of different charge and size through the core of the CNT is close to bulk diffusion expectations and allows estimation of the number of open pores or porosity of the membrane. Flux of gases such as N2, CO2, Ar, H2, and CH4 scaled inversely with their molecular weight by an exponent of 0.4, close to expected kinetic theory velocity expectations. However, the magnitude of the fluxes was ~15- to 30-fold higher than predicted from Knudsen diffusion kinetics and consistent with specular momentum reflection inside smooth pores. Polar liquids such as water, ethanol, and isopropyl alcohol and nonpolar liquids such as hexane and decane were dramatically enhanced, with water flow over 4 orders of magnitude larger than “no-slip” hydrodynamic flow predictions. As direct experimental proof for the mechanism of near perfect slip conditions within CNT cores, a stepwise hydrophilic functionalization of CNT membranes from as-produced, tip-functionalized, and core-functionalized was performed. Pressure-driven water flow through the membrane was reduced from 5 × 104 to 2 × 102 to less than a factor of 5 enhancements over conventional Newtonian flow, while retaining nearly the same pore area.
M.J. Green, C.C. Young, A.N.G. Parra-Vasquez, M.Majumder, V.Juloori, N. Behabtu, C. L. Pint, J. Schmidt, E.Kesselman, R.H. Hauge, Y.Cohen, Y.Talmon and M.Pasquali “Direct imaging of carbon nanotubes spontaneously filled with solvent” Chem. Commun., 2011, 47,4,1228–1230
For the first time, cryo-TEM imaging is used to directly show spontaneous filling of carbon nanotubes immersed in a solvent in the native state at ambient conditions. Multi-walled carbon nanotubes are dissolved in chlorosulfonic acid, and the high contrast between the acid and the carbon shows the difference between filled and unfilled nanotubes.
M. Majumder, A. Stinchcomb, B.J. Hinds, “Towards Mimicking Natural Protein Channels with Aligned Carbon Nanotube Membranes for Active Drug Delivery” Life Sciences, 86, 15-16, 2010, 563-68
Aims: Carbon nanotube (CNT) membranes offer an exciting opportunity to mimic natural protein channels due to 1) a mechanism of dramatically enhanced fluid flow 2) ability to place ‘gatekeeper’ chemistry at the entrance to pores 3) the ability for biochemical reactions to occur on gatekeeper molecules and 4) an ability to chemically functionalize each side of the membrane independently. Main methods: Aligned CNT membranes were fabricated and CNT pore entrances modified with gatekeeper chemistry. Pressure driven fluid flow and diffusion experiments were performed to study the mechanisms of transport through CNTs. Key findings: The transport mechanism through CNT membranes is primarily 1) ionic diffusion near bulk expectation 2) gas flow enhanced 1–2 orders of magnitude primarily due to specular reflection 3) fluid flow 4–5 orders of magnitude faster than conventional materials due to a nearly ideal slip-boundary interface. The transport can be modulated by ‘gatekeeper’ chemistry at the pore entrance using steric hindrance, electrostatic attraction/repulsion, or biochemical state. The conformation of charged tethered molecules can be modulated by applied bias setting the stage for programmable drug release devices. Significance The membrane structure is mechanically far more robust than lipid bilayer films, allowing for large-scale chemical separations, delivery or sensing based on the principles of protein channels. The performance of protein channels is several orders of magnitude faster than conventional membrane materials. The fundamental requirements of mimicking protein channels are present in the CNT membrane system.
M. Majumder, C. Rendall, M. Li, A. Eukel, H.K. Schmidt, M. Pasquali, “Insight into the physics of spray-coating of SWNT films” Chem.Engg.Sci.., 65, 6, 2010, 2000-08
Spray coating is a scalable and high-throughput process for fabrication of transparent and conducting coatings (TCCs) composed of single-walled carbon nanotubes (SWNTs). Presently the fundamentals of this process are not well understood. We show that suppression of coalescence of spray droplets by sufficiently rapid heat- and mass-transfer yields homogeneous SWNT films by preventing the formation of ‘coffee stains’ of larger length scale. Such heat and mass transfer is driven by differential evaporation between the top and edges of the drops, whereas thermal and compositional effects on surface tension and buoyancy are weak. Ultrasonic spraying ensures that the droplets are deposited without significant splashing, and delayed splashing at higher Weber number is evidenced. We find that the performance of spray-coated TCCs made from HiPCO SWNTs is limited by bundle diameter rather than length of the constituent SWNTs and bundes. Vapor acid doping with concentrated sulfuric acid roughly doubles the conductivity of the TCCs
M. Majumder, K. Keis, J. Cole, C. Meadows, X. Zhan, B.J. Hinds, “Enhanced Electrostatic Modulation of Transport Through CNT Membrane by Diazonium Grafting Chemistry” J. Memb. Sci., 2008, 316, 1-2, 89-96.
A membrane structure consisting of an aligned array of open ended carbon nanotubes (~7 nm i.d.) spanning across an inert polymer matrix allows the diffusive transport of aqueous ionic species through CNT cores. The plasma oxidation process that opens CNTs tips inherently introduces carboxylic acid groups at the CNT tips, which allows for a limited amount of chemical functional at the CNT pore entrance. However for numerous applications, it is important to increase the density of carboxylic acid groups at the pore entrance for effective separation processes. Aqueous diazonium-based electrochemistry significantly increases the functional density of carboxylic acid groups. pH dependent dye adsorption–desorption and interfacial capacitance measurements indicate 5–6 times increase in functional density. To further control the spatial location of the functional chemistry, a fast flowing inert liquid column inside the CNT core is found to restrict the diazonium grafting to the CNT tips only. This is confirmed by the increased flux of positively charged Ru(bipy)+23 with anionic functionality. The electrostatic enhancement of ion diffusion is readily screened in 0.1 M electrolyte solution consistent with the membrane pore geometry and increased functional density.
M. Majumder, X. Zhan, R. Andrews, B.J. Hinds “Voltage Gated Carbon Nanotube Membranes” Langmuir , 2007, 23, 8624-8631.
Membranes composed of an array of aligned carbon nanotubes, functionalized with charged molecular tethers, show voltage gated control of ionic transport through the cores of carbon nanotubes. The functional density of tethered charge molecules is substantially increased by the use of electrochemical grafting of diazonium salts. Functionality can be forced to occur at the CNT tip entrances by fast fluid flow of an inert solvent through the core during electrochemical functionalization. The selectivity between Ru(bi-pyridine)32+ and methyl viologen2+ flux is found to be as high as 23 with -130 mV bias applied to the membrane as the working electrode. Changes in the flux and selectivity support a model where charged tethered molecules at the tips are drawn into the CNT core at positive bias. For molecules grafted along the CNT core, negative bias extends the tethered molecules into the core. Electrostatically actuated tethers induce steric hindrance in the CNT core to mimic voltage gated ion channels in a robust large area platform.
M. Majumder, N. Chopra, R. Andrews, B.J. Hinds, “Nanoscale Hydrodynamics: Enhanced Flow in Carbon Nanotubes”, Nature, 2005, 438, 3, 44
Nanoscale structures that could mimic the selective transport and extraordinarily fast flow possible in biological cellular channels would have a wide range of potential applications. Here we show that liquid flow through a membrane composed of an array of aligned carbon nanotubes is four to five orders of magnitude faster than would be predicted from conventional fluid-flow theory. This high fluid velocity results from an almost frictionless interface at the carbon-nanotube wall.
M. Majumder, N. Chopra, B.J. Hinds, “Effect of Tip Functionalization on Transport through Vertically Oriented Carbon Nanotube Membranes”, J. Amer. Chem. Soc., 2005,127, 9062-70.
Ionic flux through a composite membrane structure, containing vertically aligned carbon nanotubes crossing a polystyrene matrix film, was studied as a function of chemical end groups at the entrance to carbon nanotubes’ (CNTs) cores. Plasma oxidation during the membrane fabrication process introduced carboxylic acid groups on the CNTs’ tips that were modified using carbodiimide mediated coupling between the carboxylic acid and an accessible amine group of the functional molecule. Functionalization molecules included straight chain alkanes, anionically charged dye molecules, and an aliphatic amine elongated by polypeptide spacers. Functionalization was confirmed by FTIR spectroscopy, and areal functional density was estimated by transmission electron microscopy studies of thiol terminated sites decorated by nanocrystalline gold. The transport through the membrane of two different sized but equally charged molecules (ruthenium bipyridine [Ru-(bipy)32+] and methyl viologen [MV2+]) was quantified in a U-tube permeation cell by UV−vis spectroscopy. Relative selectivity of the permeates varied from 1.7 to 3.6 as a function of tip-functionalization chemistry. Anionic charged functional groups sharply increased the flux of the cationic permeates. This effect was reduced at higher solution ionic strength consistent with shorter Debye screening length. The observed selectivities were consistent with a hindered diffusion model with functionalization at the CNT tip and not along the length of the CNT core.
N. Chopra, M. Majumder, B.J. Hinds, “Bi-functional Carbon Nanotubes by Sidewall Protection”, Adv. Funct.Mat., 2005, 15,858-64.
A vertically aligned array of multiwalled carbon nanotubes (MWCNTs) impregnated with polystyrene is utilized to independently functionalize each end of the MWCNTs. The presence of the polystyrene matrix prevents sidewall oxidation of the CNTs, resulting in carboxylate deri vatization at the CNT tips during processing via plasma oxidation. The membrane is subsequently dissolved in toluene, resulting in a suspension of CNTs with carboxylate-derivatized tips. The CNT tips are further functionalized using carbodiimide-mediated linking of carboxylate at the CNT tips with an amine of 2-aminoethanethiol. This treatment results in thiol functionality and Fourier-transform infrared (FT-IR) studies confirm amide-bond formation. Gold nanoparticles that are readily observed using transmisison electron microscopy (TEM) are then covalently linked to the thiol functional groups. Estimates of the average nanoparticle density are observed to decrease from ~526 particles μm-1 near the CNT tips to negligible values (< 7 particles μm–1) at locations beyond 700 nm from the CNT tips. This is consistent with a membrane geometry where CNTs tips are above the polystyrene surface owing to differing oxidation rates. Bifunctional CNTs (with different chemical functionality at either end of each CNT) is achieved by thiol functionalization on only one side of the oxidized CNT membrane floating on top of a 2-aminoethanethiol functionalization reaction solution. After dissolution of the polystyrene matrix, TEM analysis shows gold-nanoparticle decoration at the thiol-functionalized end of the CNT
M. Majumder, S. Mukhopadhyay, O.Parkash, D.Kumar, “Sintering and Crystallization Behavior of Chemically Prepared Cordierite for Application in Electronic Packaging”, Ceram. Inter., 2004, 30, 1067-1070.
Synthesis and sintering of dense cordierite ceramics, prepared from magnesium and aluminium ions precipitated in dispersed silica have been reported and phase evolution at 1200 °C been studied by XRD. Densification of compacted precursor powder at 1200 °C has been substantiated by SEM and it was found that the dielectric properties of cordierite ceramics prepared by this route are good enough for substrate application.